C–H Functionalization
C–H functionalization has been called1 the holy grail of synthetic organic chemistry. Recent efforts across organic chemistry, organometallics, and catalysis have made serious inroads in both understanding the reactivity of C–H bonds and developing robust reactions taking advantage of this insight, suggesting that the time is right to widely introduce these tactics to the
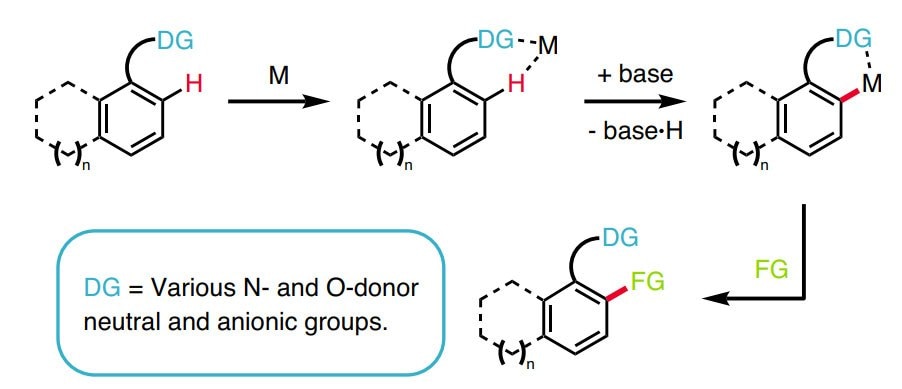
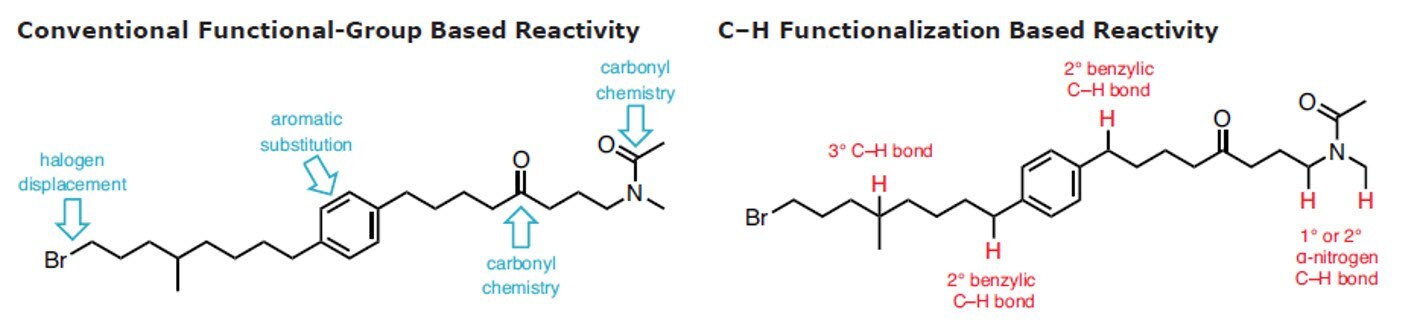
retrosynthetic lexicon.2-11 The reliable and predictable conversion of a C–H into a C–C, C–N, C–O, or C–X bond in a selective and controlled way is beneficial in terms of step economy and waste reduction.
Novel methods for C–H activation extend the number of sites that can be targeted in a given molecule, increasing the opportunity to elaborate it into a more complex product. In addition, it allows for completely different kinds of chemical bonds to be targeted in organic synthesis, particularly with high chemoselectivity. Combined with traditional functional-group chemistry, C–H functionalization considerably streamlines chemical synthesis for the construction of complex natural products and pharmaceutical compounds. While clearly there are advantages to the application of C–H functionalization logic,12 many curricula for organic chemistry have not yet been updated to reflect this approach and further information can be found in the C-H Functionalization Manual.
Related Technical Articles
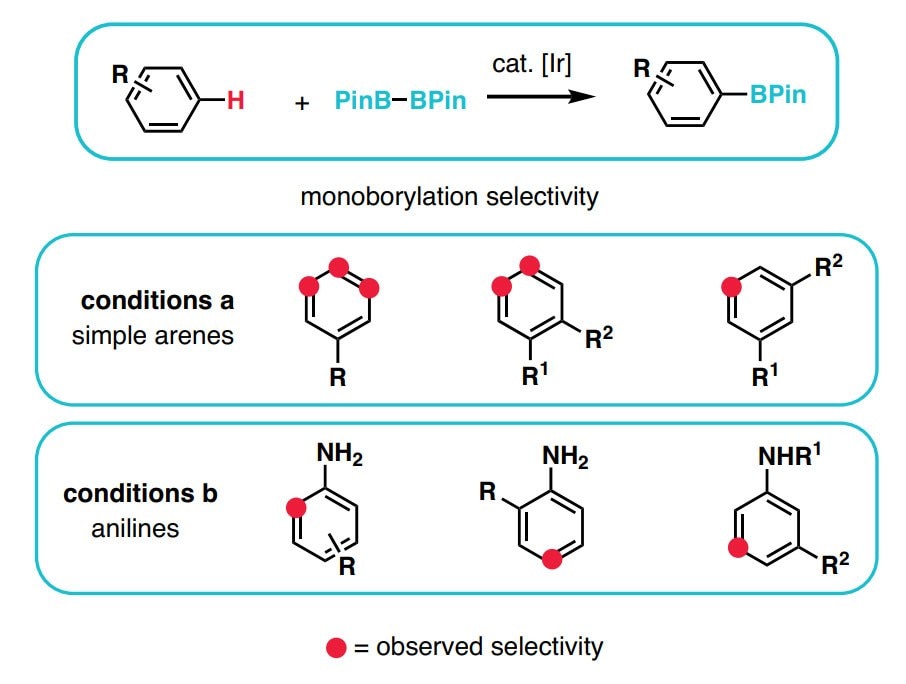
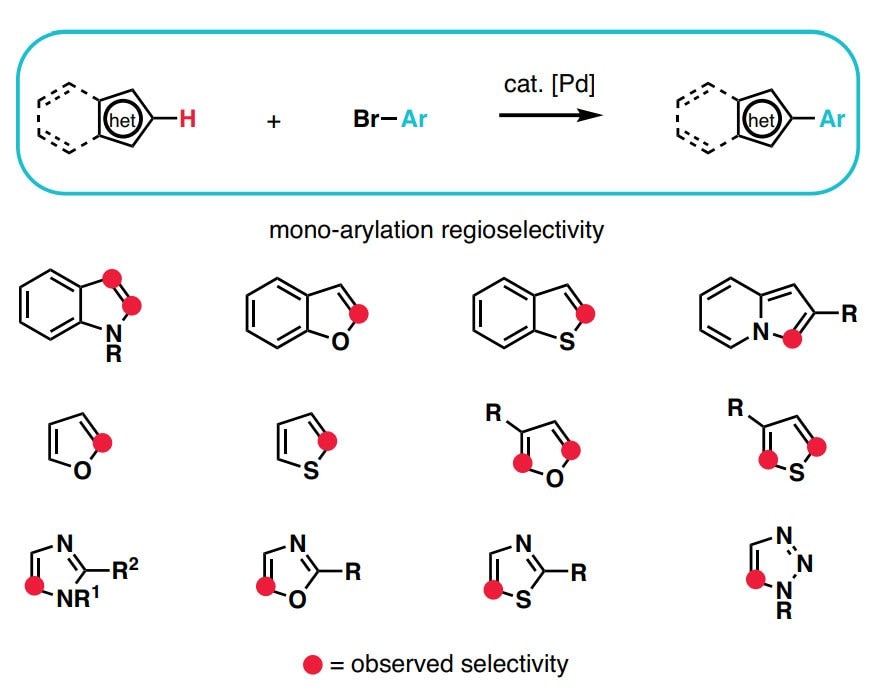
- The synthesis of heteroaromatic and aromatic compounds is at the heart of the chemical industry. The ever-growing demand for new chemical entities, coupled with dwindling resources and time constraints allotted to any given research project, a rapid way to diversify (hetero)aromatic scaffolds is needed.
- The Du Bois group at Stanford University has made substantial progress within the field of Rh-catalyzed C–H amination via oxidative cyclization of carbamate, sulfamate, sulfamide, urea, and guanidine substrates to give 1,2- and 1,3-heteroatom motifs masked in the form of 5- and 6-membered ring heterocycles.
- Professor Karl Anker Jørgensen and his group have developed ethers which serve as excellent chiral organocatalysts in the direct asymmetric α-functionalization of aldehydes.
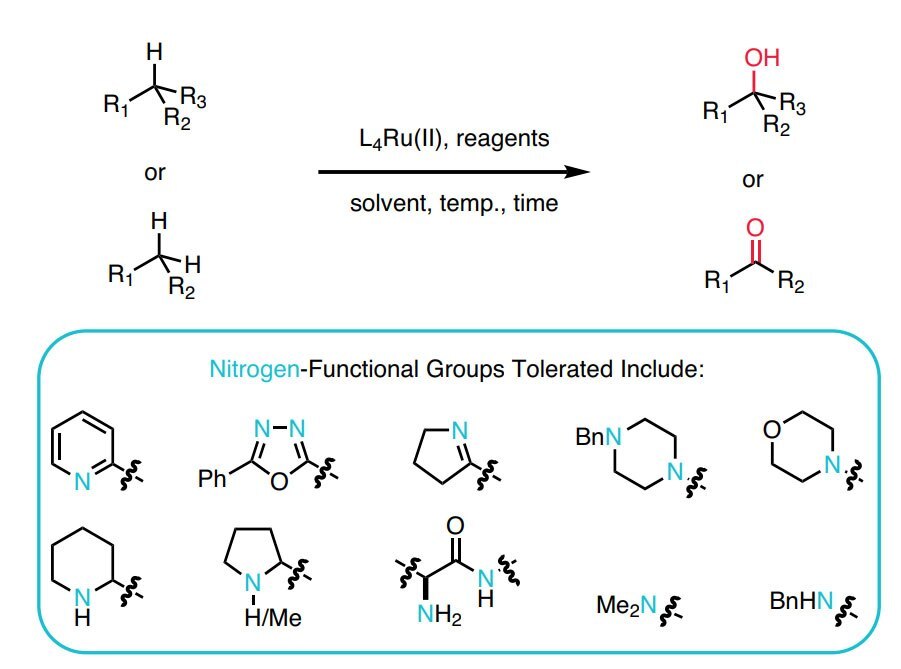
- A recyclable, ligand-free ruthenium catalyst for C–H activation reactions and concomitant C–C bond formation in the presence of water.
- Aryl chlorides are commonly used in cross-coupling reactions and can serve as key intermediates towards the synthesis of pharmaceutical drug candidates and natural products.
- See All (10)
References
To continue reading please sign in or create an account.
Don't Have An Account?