Built to Serve Pioneers
There’s a new name in CDMO with proven experience. With Millipore® CTDMO Services we simplify bringing
Millipore® Contract Testing, Development, and Manufacturing Organization (CTDMO) Services
Modalities where we have focused leadership are mAbs, ADC, HPAPI, VV, mRNA & LNP formulation
Our integrated Millipore® CTDMO Services is combining our expertise in development, manufacturing, and contract testing.
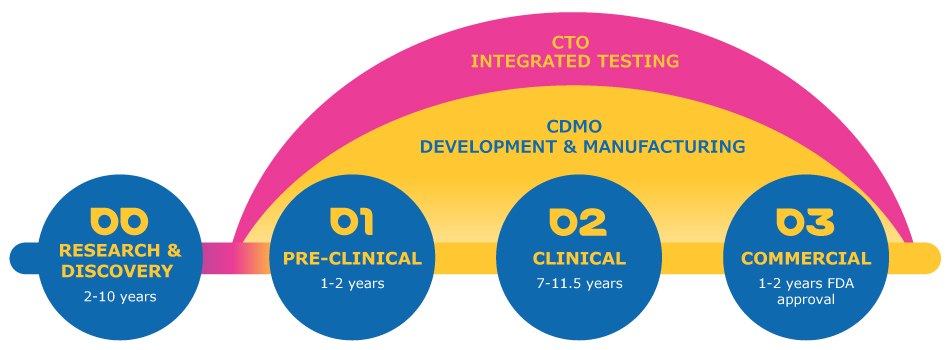
Designed to support clients seeking a single partner who can leverage technical leadership, we provide a streamlined experience that covers the entire value chain from pre-clinical to commercial.
We are united with our clients around a single goal, to help patients, by forging partnerships aimed at bringing their pioneering breakthroughs to life.
Our contract testing services are integrated into the modality services to further streamline their development path. Our expertise, speed and agility is what makes the difference for your innovations.
We leverage 30+ years of global success working with drug development, material science and process technology aligned with our supply network to accelerate your program. We are the industry’s experienced choice, built to serve pioneers.
Our CTDMO Services are Focused on the Modalities with the Most Patient Impact
Monoclonal Antibodies (mAbs) have matured to become a core therapeutic modality, with more than 128 mAbs now approved for a wide range of diseases. Our highly experienced experts will help you to optimize, scale, and validate your manufacturing process to get you to late clinical and commercial success.
Antibody-drug conjugates (ADC), known as “magic bullets”, have ignited a new era of targeted cancer therapy. With more than 35 years of experience, we are a leading expert in the conjugation of highly active molecules to monoclonal antibodies for the development and manufacture of ADCs.
Highly potent APIs (HPAPIs) are a powerful component in the treatment of diseases such as cancer. As one of the industry’s largest global manufacturers of HPAPIs, we have over 30 years of experience in the development and production of these complex, multi-step compounds.
Viral vectors (VVs) are used to deliver novel genetic material to cells advancing the potential to treat a wide range of diseases. With more than 25+ years of contract manufacturing experience, we have produced over 1,000 batches of virus to support gene therapy development from clinical through commercialization.
mRNA technology uses non-viral delivery systems and offers a great deal of versatility. As naked mRNA is unstable, drug delivery systems, such as lipid nanoparticles, are necessary to deliver mRNA to target cells. Our integrated CDMO capabilities include the development and manufacture of custom mRNAs and formulation of lipid nanoparticles (LNP).
Learn more about our small molecule services supporting Complex API and Activated PEGs

Why Contract Testing Services with your CDMO Offering Matters
Our clients manage multiple partners, supply chain needs, and complex priorities along the drug development life cycle. Our CTDMO services provide a unified and simplified experience covering the entire value chain making us your one trusted partner.
We are the Industry’s Experienced Choice
We industrialize science by standardizing manufacturing templates.
We utilize process technology and consumables that are designed to create efficient and effective processes tailored to clients’ needs.
We meet clients where they need us. We are a global organization, making significant investments in our talent and capabilities.
Our approach allows us to simplify complex supply chain needs within our extensive services network, enabling a truly integrated solution for clients.
We apply regulatory expertise gained over decades to inform and navigate clients’ pathways to approval. Our global know-how supports quality standards to ensure compliance where it matters.
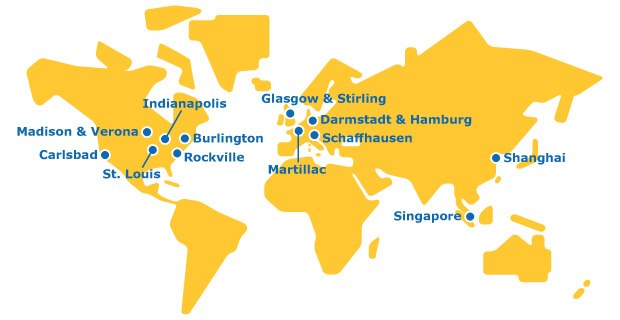
The Global Footprint of Our CTDMO Manufacturing Sites
We are a single organization with a global network to deliver CTDMO services across all stages of the molecule value chain.
Europe

Our mRNA manufacturing site for our integrated CDMO capabilities includes the development and manufacturing of custom mRNAs.

Biopharma development and manufacturing facility for mAbs production with over 25+ years of GMP experience. Including pilot, and GMP capabilities.

Our site for the manufacturing of lipid nanoparticles. Our synthetic cholesterol product offers high purity, scalability, and consistent quality.

Testing service site including product characterization, cell banking, cell line characterization, viral clearance, and lot release for our integrated CTDMO.
Asia

Biopharma development and manufacturing facility for mAbs with pilot and GMP capabilities.

One of our viral clearance service facilities for our integrated CTDMO.

Testing service site including cell line characterization, viral clearance, and lot release for our integrated CTDMO.
To continue reading please sign in or create an account.
Don't Have An Account?


















