Diagnostic OEM & CMO Services
Your Trusted Clinical Diagnostics Contract Manufacturing Partner
Our quality systems and regulatory expertise combined with a full range of custom and contract manufacturing services provide a global solution for the diagnostics industry with a focus on customer satisfaction. We are proud to partner with the world’s largest diagnostics manufacturers as well as helping new diagnostics companies navigate compliance requirements and launch the products that will transform healthcare. We offer a comprehensive array of services to meet the development and production needs of medical device manufacturers and diagnostic organizations worldwide.
Related Product Resources
Diagnostic Manufacturing Case Studies:
Case Study: IVD Case Study: CRISPR-based Assay from Mammoth Biosciences
Case Study: Elypta Needed Contract Manufacturing for a Novel Metabolism-Based Liquid Biopsy
Guides to Faster IVD Commercialization:
10 Strategic Considerations When Outsourcing Production of InVitro Diagnostics
Supply chain considerations when developing or manufacturing your IVD assay.
Quality & Regulatory Management, ISO Certifications, and more...
Considerations When Developing Diagnostic Assays Using Leading-Edge Technologies
Tech Article: Vetting a Contract Manufacturing Partner for Your Clinical Diagnostics Kit
Tech Article: Accelerating Time to Market Using Custom Lateral Flow Membranes
Technical Articles for Diagnostic Assay Development:
Brochure: Contract Manufacturing Capabilities
Tech Article: Complete Solutions for IVD Chemiluminescent Immunoassay (CLIA/CLEIA) Development
Tech Article: Complete Solutions for PCR Assay Development
Image 1.Key technologies we offer for diagnostic assay OEM and Contract Manufacturing Services
Key Technologies
We offer extensive expertise in many technologies needed to successfully commercialize medical devices and IVD assays.
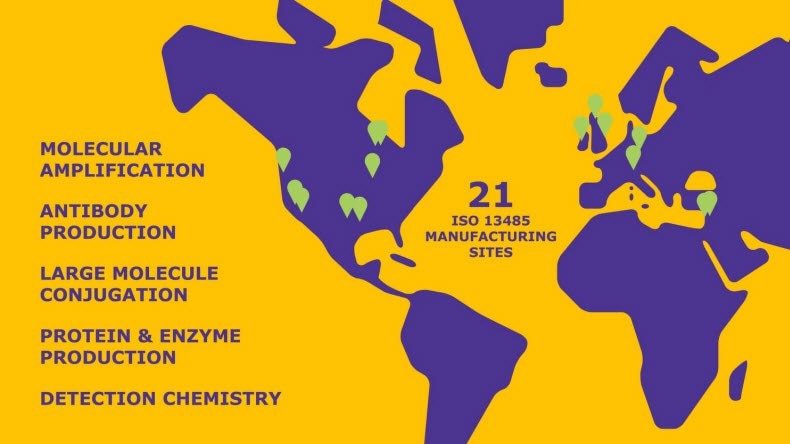
Molecular Amplification
Conjugation
|
Bio-organic Reagents
Detection Chemistry
|
Antibody Production
Protein/Enzyme Production
|
Packaging Capabilities
We have extensive manufacturing capabilities with a secure supply-chain and global footprint to give you the reliability, consistency, and flexibility to meet your needs.
- Sterile filtration
- Hazardous materials
- Automated torque and weight checks
- Environmentally-controlled suites
- Molecular biology-grade environments
Powder Fill
- 10 ug to 200 kg+
- Multiple sealing and capping options
- Pouching and tableting
Liquid Fill
- Automated filling capabilities
- Large batch volume for IVD-grade liquid solutions
- 10 µl to 20 L fill volumes
- Large contract lyophilization capacity
- Environmentally-controlled filling
Formats
- Glass and plastic bottles
- Tubes and vials
- Boxes and Bags
- Cubitainers™
- Large Volume Totes
- Multiwell plates
- Custom labeling including Barcoding
Custom QC Testing
Image 2.QC testing analysis for an OEM or Contract Manufacturing Services for diagnostic assay
We offer key QC assay formats relevant to your needs.
Our analysis capabilities include:
Molecular - qPCR, PCR, western blotting, Southern blotting, electrophoresis, nuclease contamination, bacterial growth, cell component isolation kits (DNA, RNA, protein)
Immunological - ELISA, western blotting, Ouchterlony double diffusion, cell staining, histology, serology
Functional - Plasmid purification, cloning, protein depletion, proximity ligation assays (Duolink®)
Other specialized analyses
- Endotoxins, hematology, enzymatic activity and contamination, cell culture, tablet testing (hardness, friability, and dissolution)
- Test method validation for niche QC needs and ability to partner with external testing labs for esoteric tests
- Functional testing of contract manufactured products on client platform
Commitment to Quality
We are an industry leader for complex manufacturing our quality systems are designed to meet stringent regulatory requirements including:
- ISO 9001:2015
- ISO 13485:2016
- 21 CFR 820
- 98/79 EC In vitro Diagnostics Directive
- ISO 14001:2015
Our global footprint includes 21 ISO 13485 manufacturing sites.
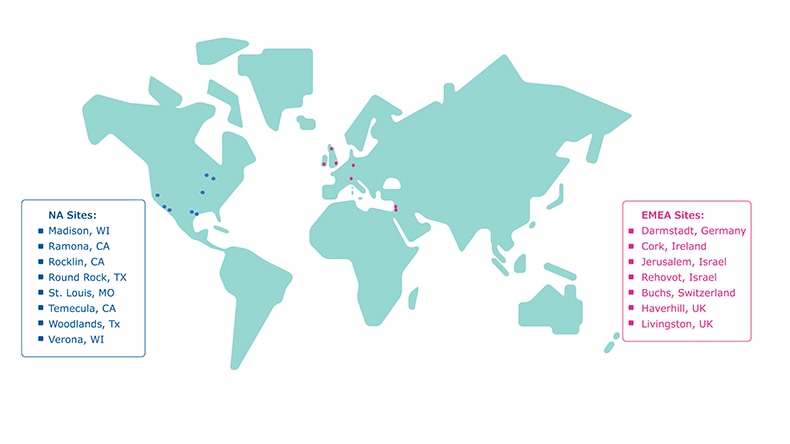
Image 3.NA and EMEA manufacturing sites for diagnostic assay components
Experienced Project Management to Secure Success
Our dedicated project managers are scientists who understand the technical, operational, and regulatory challenges of our client’s projects. For each client, we offer:
- Single Point of Contact for operations and project management
- Resources to help you navigate industry guidelines and regulations
- Support through your project life cycle
Steps for Success
Inquiry and Consultation
After receiving your inquiry, you will be contacted by one of our Business Development Specialists.
Acceptance
Project specifications are reviewed internally to determine feasibility and costing. Quote for project sent to you.
Creation of Project Team/Implementation
A cross-functional project team is assembled with expertise specific to project. Small-scale validation batched produced.
Full-Scale Implementation
We manufacture at scale to your specifications with your custom packaging/labeling for regional or global distribution.
To continue reading please sign in or create an account.
Don't Have An Account?