Blazar® Rapid Molecular Detection
Traditional testing approaches for biologics such as mAbs, vaccines as well as emerging cell and gene therapies can take several weeks. Indeed, these traditional in vivo or in vitro methods may also be incompatible with the unique challenges presented by your therapy.
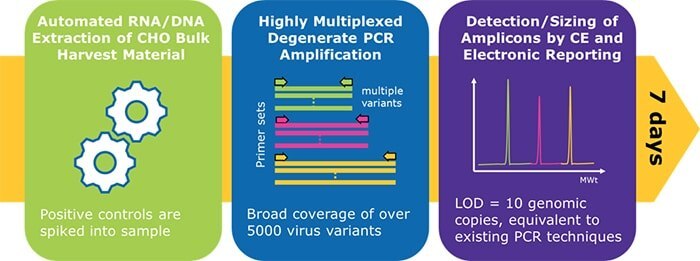
The Blazar® platform provides researchers with accurate and highly sensitive viral detection in just days. Combining the breadth of detection of next generation sequencing (NGS) with the speed and sensitivity of PCR methods, the Blazar® platform relies on a degenerate PCR approach for coverage of more than 5,000 viral variants. By amplifying multiple targets within a conserved region of the viral family genome, you can detect a much broader range of adventitious viruses than with traditional PCR methods.
- Fast – screen for viruses within days and release your batches faster
- Robust – sample directly from the bioreactor with no sample clean-up
- Broad coverage – detect viral variants using degenerate primers
- Sensitive – validated sensitivity of 10 genomic copies, even in challenging matrices
- Small sample volume – conserve precious material
- Automated – minimize handling errors
- Digital assessment and reporting – traceable and accurate
- Enables continuous manufacturing – expedite biomanufacturing timelines
A viable alternative to in vitro detection assays
The Blazar® platform reduces dependence on in vivo models for virus detection. By replacing the mouse, hamster, and rat antibody production (MAP, HAP, and RAP) tests typically used to characterize rodent cells for biologics manufacturing, assays can instead champion the 3Rs (replacement, reduction, and refinement) while meeting both corporate and regulatory imperatives to decrease animal use.
Bulk harvest lot release testing (BHLRT) can be a biomanufacturing bottleneck, taking several weeks with the many regulatory-required analyses. While there have been innovations in biosafety testing, in vitro virus detection has remained the same until now. The Blazar® rapid molecular detection platform.
The Blazar® rapid molecular platform is a viable alternative to in vitro detection assays
The Blazar® platform will enable next generation bioprocessing strategies – from process intensification, to connected processing, to fully continuous manufacturing – expediting biomanufacturing timelines thorough enhanced productivity. Used in combination with our BioContinuum™ Platform, the Blazar® platform offers significant potential to revolutionize biologics production.
Maintain a competitive advantage by accelerating your biologics program with the Blazar® platform for rapid molecular detection.
Use the form below to learn more about maintaining a competitive advantage and accelerating your biologics program with the Blazar® platform.
To continue reading please sign in or create an account.
Don't Have An Account?