A Fast and Reliable Method for Purity Analysis of Filgrastim
Cory E. Muraco
Reporter US Volume 34.2
Introduction
In recent years, there has been a large increase in the development of biological-based therapeutics, known as biologics, to treat a myriad of diseases. In 2015, biologics comprised 20% of the pharma market, and most analysts agree that this trend will continue to increase in the years to come.1 Biologic drugs generally are proteins, antibodies, or modified forms of biomolecules, such as an antibody conjugated to a small, organic molecule. One protein that is used in biologics is filgrastim. Filgrastim is a recombinant form of human granulocyte colony stimulating factor (GCSF) produced in Escherichia coli bacteria. Filgrastim is used to treat neutropenia, a disease characterized by an abnormally low concentration of neutrophils (white blood cells) in the blood.2
Since filgrastim is used in different biologics, it is crucial for the purity of filgrastim to be known. The main impurity present in many formulations of biologics that contain filgrastim are oxidized variants of the protein. Filgrastim has four methionine residues present in its primary structure and since methionine can be oxidized to methionine sulfoxide, there could theoretically be up to 24 different oxidized variants present in a single formulation.3 The United States Pharmacopeia (USP) has a monograph outlining a reversed-phase separation protocol for the detection and quantitation of these oxidized variants; however, the protocol uses a large diameter (4.6 mm) high performance liquid chromatography (HPLC) column, a 35 minute mobile phase gradient, and a large (33 μL) sample size.4 For a high-throughput quality control (QC) lab, this method would use a large amount of sample, mobile phase, and time; all of which would increase the costs of operating the lab. It is desirable to develop a fast, reliable method that minimizes sample size, mobile phase consumption, and run time while not compromising the efficiency of the USP method. Such a method was developed using the BIOshell A400 Protein C4 column.
Experimental Conditions
The conditions used in the USP method are given in Figure 1.
The conditions used in the optimized method are given in Figure 2. The oxidized filgrastim sample was prepared by diluting a 2 mg/mL stock solution to 0.75 mg/mL (USP specifications) or 98 μg/mL (to prevent sample overload in smaller I. D. column) with 0.1% hydrogen peroxide. The oxidation reaction occurred at room temperature for five hours prior to assay.
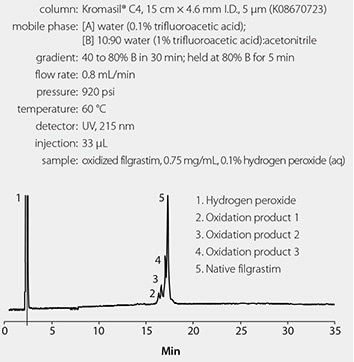
Figure 1. Analysis of Oxidized Filgrastim Using the USP Method
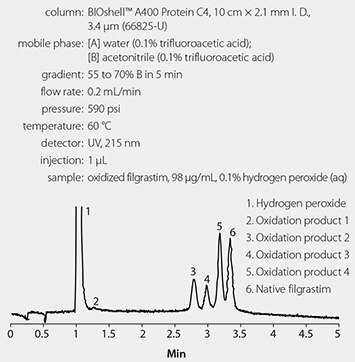
Figure 2. Analysis of Oxidized Filgrastim Using an Optimized Method
Results
Figure 1 shows the results of the chromatographic analysis of oxidized filgrastim using the current USP monograph method, and Figure 2 shows the results of the chromatographic analysis of oxidized filgrastim using the optimized method. Resolution between the different oxidation products and between the oxidation products and native filgrastim is poor using the USP method; whereas resolution is significantly improved using the optimized method. In addition, a fourth oxidation product (Peak 2 in Figure 2) is observed in the chromatogram that is not observed using the USP method. Finally, run times have been reduced to less than five minutes using the optimized method, whereas the USP method requires a 35 minute run time.
Conclusion
The determination of the purity of a biologic is of the utmost importance to a pharmaceutical QC lab. Both the USP method and the optimized method can tell a scientist or technician if there are impurities in a sample of filgrastim. However, the optimized method can perform this analysis with better resolution and sensitivity than the USP method, and it also reduces run times 7-fold, solvent consumption 28-fold, and sample amount 250-fold, relative to the USP method, thus making it a more cost-effective method than the USP method.
References
To continue reading please sign in or create an account.
Don't Have An Account?