A Fluorescence Derivatization Reagent for the Liquid Chromatographic Determination of Airborne Isocyanates
Hartmut Henneken, Uwe Karst, Martin Vogel
Faculty of Science and Technology and MESA+ Institute for Nanotechnology, University of Twente, Enschede, The Netherlands.
Reporter EU Volume 13
Currently, mono- and diisocyanates are widely applied for the industrial production of pharmaceuticals, pesticides or polyurethanes (PURs). Owing to their high reactivity, isocyanates are toxic. Isocyanates in general have strong sensitising properties and are supposed to be most common inducers of occupational asthma. Furthermore, exposure to mono- or diisocyanates is prone to cause irritations of the respiratory system, which may lead to acute pulmonary oedema. Therefore, characterisation and sensitive quantification of this class of analytes is important in the fields of workplace analysis.
Today, modern analytical approaches for the determination of isocyanates base on the derivatization with nucleophilic reagents such as alcohols or secondary amines. The derivatization is followed by a chromatographic separation with mostly photometric or fluorimetric detection. Currently, a number of different derivatizing agents are available. However, only few of them offer sufficient sensitivity, for example, 9-(N-methylaminomethyl)-anthracene (MAMA)1 or dibutylamine (DBA).2 Although for MAMA, HPLC detection can be done both photometrically or fluorimetrically, absorption maxima in the short-wavelength range of the electromagnetic spectrum may lead to interferences from matrix constituents. With respect to DBA, the application is restricted to mass spectrometric detection as the reagent is lacking any chromophoric or fluorophoric group.
In analytical chemistry, derivatizing agents based on the 2,1,3- benzoxadiazole backbone (Figure 1) have found increasing application mainly for bioanalytical or environmental purposes. Although all benzoxadiazoles are likely to be excellent chromophores with absorption maxima in a range > 350 nm accompanied by high molar absorptivities, fluorescence characteristics are restricted to a limited number of benzoxadiazole compounds.
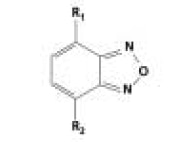
Figure 1. Structure of the benzoxadiazole (benzofurazan) backbone
Earlier, 4-nitro-7-piperazino-2,1,3-benzoxadiazole (NBDPZ) (Figure 2) had been applied to the determination of carboxylic acids.3 Making use of NBDPZ for isocyanates, however, both mono- and diiso-cyanates can be determined by means of reversed-phase (RP) liquid chromatography with subsequent photometric, fluorimetric,4 or even mass spectrometric detection.5, 6
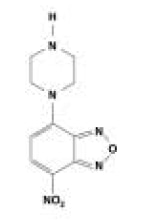
Figure 2.Structure of NBDPZ
Isocyanates readily react with NBDPZ, thus yielding the respective urea compounds. NBDPZ urea derivatives possess UV-Vis absorption maxima in the range of 480 nm, high molar absorptivities (~25,000 L·mol·cm-1 per NBDPZ moiety), and additionally, they show good fluorescence characteristics. Excitation wavelengths are at ~ 470 nm, while emission is observed at ~ 535 nm. Thus, compared to all established amine derivatizing agents, NBDPZ provides for the most red-shifted absorption maxima. Using a binary gradient consisting of an aqueous buffer and acetonitrile, reversed-phase C18 LC columns show good results for the separation of NBDPZ and its corresponding isocyanate derivatives. However, to overcome a co-elution problem of the derivatives of 2,6-toluene diisocyanate (2,6-TDI) and 1,6-hexamethylene diisocyanate (HDI or HMDI), a phenyl-modified reversed-phase column shows best chromatographic resolution.4
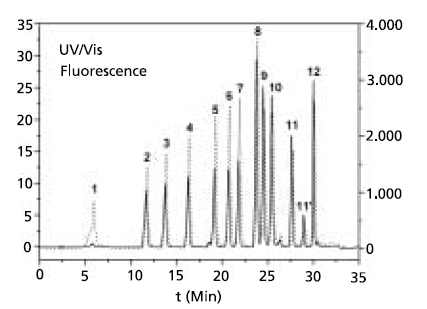
Figure 3.Liquid chromatographic separation of NBDPZ (1) and the derivatives of methyl isocyanate (2), ethyl isocyanate (3), propyl isocyanate (4), butyl isocyanate (5), pentyl isocyanate (6), phenyl isocyanate (7), 2,6-toluene diisocyanate (8), 1,6-hexamethylene diisocyanate (9), 2,4- toluene diisocyanate (10), isophorone diisocyanate (11/11’), methylene bis(phenyl isocyanate) (MDI) (12) with UV-Vis (solid line) and fluorescence detection (dotted line) on a phenyl-modified column.
Apart from spectroscopic techniques, NBDPZ and its derivatives can also be detected by means of mass spectrometry. LC-MS experiments have been run using APCI(+) tandem MS5 as well as APCI(-) with single quadrupole detection.6
Using NBDPZ as derivatization reagent, the analysis of gaseous samples can either be done by active (pumped)4 or by means of passive (diffusive) sampling methods.5 Active methods comprise the use of impingers filled with NBDPZ dissolved in an organic solvent as well as the application of reagent-coated test tubes. Diffusive sampling devices - NBDPZ-impregnated filters in a dedicated housing - have been tested for the determination of methyl isocyanate and turned out to be useful for the monitoring of long-term exposure at workplaces.
Summary
NBDPZ can ideally be applied as a reagent for the determination of mono- and diisocyanates in air samples. Due to versatile spectroscopic properties, selectivity of this method is superior to other reagents. The reagent and the respective urea derivatives can be separated by means of reversed-phase HPLC. Here, highest selectivity is obtained on phenyl-modified phase. The HPLC separation can be followed by fluorescence, UV-Vis or MS detection. Regarding sampling procedures, pumped as well as passive methods are successfully applied.
Literature
To continue reading please sign in or create an account.
Don't Have An Account?