Analysis of Bisphenol A and Analogous Compounds in Infant Formula
Olga I. Shimelis, Principal R&D Scientist, K. G. Espenschied, R&D Technician, Jennifer Claus, Non-Bio Sample Preparation Product Manager
Reporter US Volume 34.4
Introduction
Bisphenol A (BPA) monomer is widely used for manufacturing plastics and epoxies. Such plastic materials are used to line food containers and metal cans. The monomer is never fully reacted, and detectable levels of BPA are known to leach from these materials into foodstuffs that contact them. Therefore, the presence of BPA in canned goods using these materials is presumed.
BPA levels in foodstuffs used for human consumption are regulated in the US and internationally but the regulations are not uniform. For example, some agencies may, and do hold that no risk to infant health is posed by using baby bottles made with plastics containing BPA. Others have moved to legally ban the use of BPA containing materials in any products used for infants and children.1,2 Acceptable levels of BPA contamination in products used by adult consumers are also subject to regulations. The overall result is testing protocols and concentration standards pertaining to BPA in food products and packaging vary accordingly; and BPA is a major analyte of interest in many testing laboratories.
There is, however, a trend to curtail the use of BPA in plastics associated with foods. In order to manufacture plastics without BPA, manufacturers are turning to other monomers with similar structures as substitutes for BPA. Among these compounds are Bisphenol S (BPS) and Bisphenol F (BPF). Scientific work indicates these compounds may be similar to BPA in terms of adverse human health effects. For this reason, the EU currently sets limits for Bisphenol S migration in food contact articles.3,4 A need exists, therefore, for analytical methods capable of detecting and quantifying a range of compounds.
In this work, we explored the use of a SupelMIP® SPE - Bisphenol A cartridge for the extraction of five related compounds from infant formula. Molecularly imprinted polymers (MIPs) are designed for the selective extraction of specific compounds or classes of compounds from various matrices. The polymeric sorbents in these SPE cartridges contain areas of functionality created during the polymerization process designed to retain a molecule with a specific chemical structure. In the case of compounds with highly similar structures, cross-selectivity is possible. An SPE cartridge designed to select for BPA may also specifically bind analogous compounds such as Bisphenol F and others.
Experimental
Five compounds were chosen for this work: BPA, BADGE, BPC, BPF, BPG. Chemical nomenclature and CAS numbers are included in Table 1.
The premixed liquid infant formula sample was spiked with a mixture of BPA compounds at 10 ppb. A 5 mL volume of sample was mixed with 5.5 mL of acetonitrile and centrifuged at 4500 rpm for 10 min in a polypropylene centrifuge tube. The top layer was collected and 3 mL of the top layer was diluted with 3 mL of water for use as the SPE loading sample.
The SupelMIP SPE - Bisphenol A method was modified to ensure complete recovery of all five compounds. Our work indicated it was necessary to change the elution solvent from 100% methanol to 3 mL of 3:1 methanol:acetonitrile (v/v) in order to improve recoveries for all compounds. The full description of the SPE method used is presented in Table 2.
The samples were reconstituted into 0.5 mL of 40:60 acetonitrile:water. All samples were filtered through Durapore® filter units prior to analysis. The HPLC method incorporated a Titan™ UHPLC column that provided good retention and peak separation for all tested compounds (Figure 1).
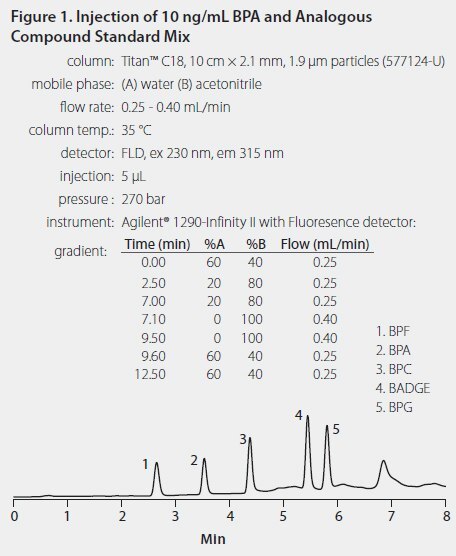
Results and Discussion
Analysis of BPA and Analogues – Elimination of Laboratory Background
Laboratory environments include a large variety of plastic materials. As a result, BPA contamination can be an issue when preparing and analyzing samples, especially when performing trace analyses. During our studies it became apparent that storage containers, auto-sampler vials, and caps can contribute to contamination, and even chromatography solvents can be contaminated. Our experience with BPA background when doing trace analysis resulted in the implementation of the following precautions as a means to reduce or eliminate environment BPA contamination:
- Reagent solutions and samples were prepared and stored in glass vials and containers
- For trace levels of studies, LC/MS grade solvents were used for sample preparation and analysis
- Vials and caps were screened for BPA prior to use
- Mixtures were gently swirled when possible, versus being vigorously shaken or vortexed to avoid contact of the liquid with the cap plastic liners
- Time during which the samples were in contact with any plastic vessels or tubes was minimized
- Sample analyses were conducted immediately after sample preparation
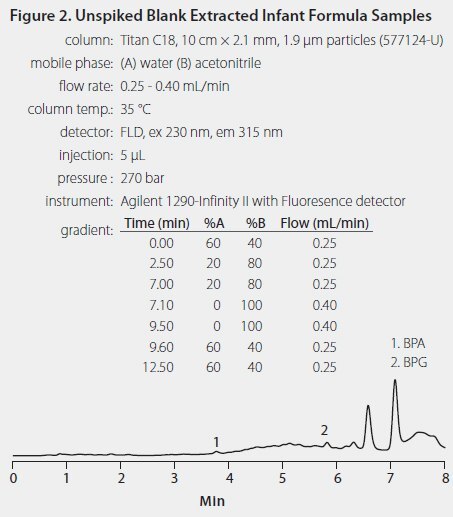
Significant BPA background arising from SPE tubes containing the sorbent was not observed. Plastic centrifuge tubes were used during the extraction step, and blank extracts did contain small amounts of BPA and BPG, possibly originating from the tubes (Figure 2.) The final results were corrected for these background values.
Recovery for BPA Compounds
The SPE procedure was first developed and tested using spiked solvent solutions. The developed method was then applied to infant formula. Most compounds showed acceptable recovery from both as seen in Table 3. BPF recovery was low from infant formula at only 41%. It was found that this compound required more polar conditions during the extraction step than the rest of the compounds. The ratio of infant formula to the acetonitrile (5 mL to 5.5 mL) provided a good compromise for accepted recoveries of BPF and BADGE, the latter requiring less polar extraction conditions. All other compounds demonstrated recovery values of 70% or higher using the developed SPE method.

Conclusion
Bisphenol A (BPA) and four structurally related compounds were extracted from infant formula using SupelMIP SPE-Bisphenol A. The results demonstrated that the same molecularly imprinted polymer (MIP) phase developed for BPA also works to extract compounds of similar chemical structure. The analysis was performed using a Titan C18 UHPLC column with fluorescence detection which gave baseline resolution for all five peaks. The samples were spiked at 10 ppb, and, with the exception of BPF, recoveries for all compounds were above 70%.
References
To continue reading please sign in or create an account.
Don't Have An Account?