Cultrex® 3-D Spheroid Fluorometric Proliferation/Viability Assay Protocol
Reagent kit for investigating spheroid cell proliferation and/or viability
Product Number 3510-096-K
Background
Current in vitro tumor models lack either a physiological context and/or reproducible format for evaluating tumor cells in vitro. At present, the most popular method for compound screening and pathway analysis involves culturing cancer cells on rigid, tissue culture treated plastic surfaces where the cells adhere non-specifically and proliferate as a monolayer, and as a result, these cells lose both morphology and gene expression profiles associated with tumors in vivo. Alternatively, single cell suspensions may be embedded in extracellular matrix (ECM) hydrogels to construct 3-D cultures; however, the resulting structures are dispersed throughout the gel and exhibit significant variability in morphology and size, limiting the establishment of physiological gradients and adversely affecting the reproducibility of each assay. To address issues of reproducibility and to build more physiological tumor systems, well-established methods for multicellular spheroid formation were incorporated into 3-D culture models. Researchers have been using spheroid cultures for cancer research for over 40 years1-3; however, there have been limitations regarding which cell lines could spontaneously form spheroids. For spontaneously spheroid assembly, it was shown that the cells produce an ECM that is deposited on the outer surface of the spheroid and that cell lines that could not spontaneously form compact spheroids were deficient or lacking in the formation of this ECM4,5. It was later shown that the addition of ECM proteins to non-spheroid forming cells induced spontaneous spheroid formation, making the spheroid format compatible with most solid cancer cell models6. Cultrex® 3-D Spheroid Fluorometric Proliferation/Viability Assay provides the necessary reagents to evaluate your cells using this method. Simply harvest cells, resuspend in spheroid formation ECM, and then culture in a 96 well spheroid formation plate. Spheroids generally form in 48 to 72 hours. Cell number and culture time determines spheroid size, and since each well produces one spheroid, researchers have complete control over spheroid dimensions with virtually no well to well variability. For most tumor models, we recommend spheroids between 400 – 500 μm in diameter. This is sufficient to establish physiological gradients for nutrients, oxygen, pH, and catabolites due to limitations in diffusion through the multicellular layers. Another effect of these gradients is the establishment of heterogeneous cell populations with necrotic cells in the core, quiescent cells in the deeper layers, and proliferating cells on the spheroid surface; all of these factors reminiscent of an avascular tumor7-10. Once formed, these multicellular tumor cell aggregates can be treated with pharmacological compounds to evaluate the effect on tumor spheroid growth; alternatively, specific genes or pathways may be manipulated to evaluate their effect on expansion of the in vitro tumor. This process can be monitored in real-time and label-free using image analysis software to measure spheroid area, and the kit is supplied with the fluorescent cell viability reagent Resazurin for quantitative end point analysis. The non-fluorescent Resazurin is reduced to fluorescent Resorufin (excitation 530-560 nm/emission 590 nm) in the mitochondria11.
Product Description
The 3-D Spheroid Fluorometric Proliferation/Viability Assay provides a useful tool for modeling tumor response in vitro. The kit utilizes 3-D Culture Qualified 96 Well Spheroid Formation Plate alongside a specialized Spheroid Formation ECM to drive aggregation and/or spheroid formation of cells. Upon completion of spheroid formation, the spheroid may be treated with pharmacological agents to evaluate tumor viability after drug treatment. Tumor spheroid expansion is visualized microscopically and can be quantitated through image analysis software for real-time and label free evaluation. At the conclusion of the assay, cell viability may be assessed by fluorescence using Resazurin. The 3-D Spheroid Fluorometric Proliferation/Viability Assay offers an in vitro, standardized, three-dimensional, high content format for inducing multicellular tumor spheroid (MCTS) formation and quantitating cell viability within the spheroids in response to pharmacological treatment.
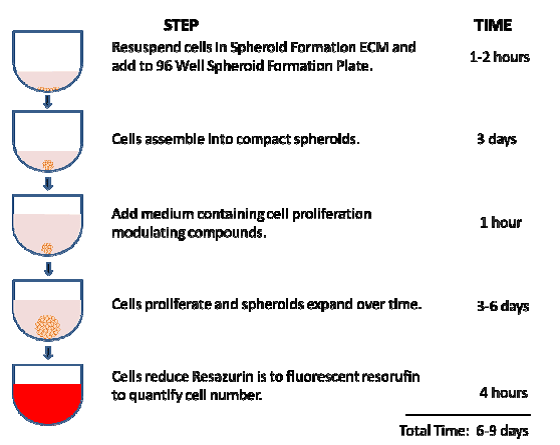
Figure 1. Steps comprising the 3-D Spheroid Fluorometric Proliferation/Viability Assay.
Components |
|---|
Reagents and Equipment Required but not Provided
- Equipment
- Laminar flow hood or clean room
- 37 °C CO2 incubator
- Low speed swinging bucket 4 °C centrifuge and tubes for cell harvesting
- Hemocytometer or other means to count cells
- –80 °C storage
- Ice bucket
- Standard light microscope (or inverted)
- Pipettes and pipette aid
- Bright Field Microscope with 4X objective and digital camera
- Timer
- Computer
- Image analysis software, such as ImageJ
- Graphing software, such as Microsoft® Excel®
- Fluorescence plate reader (excitation 530-560 nm and emission 590 nm)
- Reagents
- Cell line(s) of interest
- Cell Harvesting Buffer; EDTA, trypsin, or other cell detachment buffer
- Tissue Culture Growth Media
- Pharmacological agents for addition to culture medium, if necessary
- Phosphate buffered saline (Product No. P5493) or Hanks’ Balanced Salt solution (Product No. H9269) to wash cells
- Trypan blue (Product No. T6146) or equivalent viability stain
- Disposables
- Greiner culture flasks, tissue culture treated, 25 cm2 (Product No. C6231) or 75 cm2 (Product No. C7106)
- Centrifuge tubes, 10 ml (Product No. SIAL0790) and 50 ml (Product No. SIAL0828)
- Serological pipettes, 1, 5, and 10 ml (Product No.s SIAL1485, SIAL1487, SIAL1488)
- 1 - 200 µL (Product No. P5037) and 200 – 1000 µL pipette tips
- Gloves
Precautions and Limitations
- For Research Use Only. Not for use in diagnostic procedures.
- The physical, chemical, and toxicological properties of these products may not yet have been fully investigated; therefore, we recommend the use of gloves, lab coats, and eye protection while using these chemical reagents.
- The CULTREX® 3-D Spheroid Fluorometric Proliferation/Viability Assay contains reagents that may be harmful if swallowed, or come in contact with skin or eyes. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
Preparation Instructions
- 10X Spheroid Formation ECM
10X Spheroid Formation ECM should be thawed on ice at 4 °C and diluted with Tissue Culture Growth Medium chilled to 4 °C; pipet up and down with a serological pipet to mix. Cells are resuspended in 1X Spheroid Formation ECM, and 50 µl of cell suspension is added to each well of the 3D Culture Qualified 96 Well Spheroid Formation Plate.
See Table 1 for recommended dilution schedules. - Resazurin
Resazurin should be thawed at room temperature, and gently inverted to make a homogenous solution. Once thawed, Resazurin is stable at 4 °C for up to a month, or it may be stored in working aliquots at -20 °C for several months. - Tissue Culture Medium with Invasion Modulating Compounds
Add 2X concentration of Invasion Modulating compounds within the tissue culture medium to compensate for changes in total volume due to the spheroid embedded within the Invasion Matrix. Tissue culture medium must be 37 °C when added to gelled invasion matrix to preserve gel properties.
Storage and Stability
Product is stable for a minimum of 3 months from date of shipment when stored at –20 °C in a manual defrost freezer. For optimal stability, store at –80 °C. Avoid freeze-thaw cycles.
Assay Protocol
These procedures should be performed in a biological hood utilizing aseptic technique to prevent contamination.
A. Cell Harvesting
Culture cells per manufacturer’s recommendation. The following procedure is suggested and may need to be optimized to suit the cell type(s) being studied.
- Cells need to be healthy and proliferating prior to use in the assay. Cells should be passaged 2 or 3 times and evaluated for cell viability by trypan blue or equivalent assay. Do not start the assay until cell viability is greater than 90%.
- Each well requires approximately 2,000 - 5,000 cells, and 25 and 75 cm2 flasks yield at least 1 x 106 and 3 x 106 cells, respectively. Plan accordingly.
- Prior to harvest, visually inspect cells, and record cell health, relative number, and morphology.
- Wash cells two times with sterile PBS or HBSS. Use 5 ml per wash for a 25 cm2 flask and 10 ml per wash for a 75 cm2 flask.
- Harvest cells. For 25 cm2 flask or 75 cm2 flask, add 1 ml or 2 ml, respectively, of Cell Harvesting Buffer (see Materials Required But Not Supplied), and incubate at 37 °C for 5 to 15 minutes until cells have dissociated from bottom of flask.
- Transfer cells to a 15 ml conical tube, and add 5 ml of cell culture medium.
- Centrifuge cells at 200 x g for 3 minutes to pellet cells, remove medium, and resuspend cells in 2 ml of cell culture medium. Cells may need to be gently pipetted up and down with serological pipette to resuspend cells and break up cell aggregates. Visually inspect cells to verify formation of a single cell suspension, no aggregates prior to counting.
- Count cells and evaluate cell viability by trypan blue exclusion or equivalent test. Do not start the assay if cell viability is less than 90%.
- Dilute to 1 x 106 cells per ml in cell culture medium.
B. 3-D Spheroid Fluorometric Proliferation/Viability Assay
- Assay Preparation – Prior to Day 0
- Establish assay parameters and appropriate controls. Appropriate controls include samples with and without proliferation/viability modulating agents, as well as samples without cells for background determination.
- Determine optimal seeding density for each cell line used. In general, 3,000 cells per well is a good starting point. Optimal seeding density can be evaluated by analyzing the size of spheroid formation using the Spheroid Formation protocol (VIII.A.). Serial dilutions of cells may be placed in each well to create a standard curve; this may be useful in evaluating spheroid size and linearity of Resazurin fluorescence curve (IX.3.).
- Culture cells per manufacturer’s recommendation; adherent cells should be cultured to no greater than 80% confluence.
- Thaw 10X Spheroid Formation ECM on ice overnight in a 4 °C refrigerator.
- Spheroid Formation – Day 0
- Harvest and count cells, as directed in section VIII. A.
- Prepare a single cell suspension in 1X Spheroid Formation ECM. See section VI.1. for reagent preparation
- Dispense 50 μl of the single cell suspension in 1X Spheroid Formation ECM per well of the 3D Culture Qualified 96 Well Spheroid Formation Plate.
- Centrifuge at 200 x g for 3 minutes at room temperature in a swinging bucket rotor.
- Incubate at 37 °C in a tissue culture incubator for 72 hours to promote spheroid formation.
- 3D Culture Spheroid Treatment – Day 3
- Add 50 μl of warm (37 °C) cell culture medium containing proliferation/viability modulating compounds, if applicable. See section V.3 for reagent preparation.
- Incubate the plate at 37 °C in a tissue culture incubator for 3 to 6 days, and photograph the spheroid in each well every 24 hours using the 4X objective. Adjust the lighting and focus to provide the most contrast between the 3D structure and background. The use of fluorescence microscopy for cells that express fluorescent protein or have a fluorescent label may also improve contrast and subsequent analysis. Debris may transfer to the bottom of the 96 well plate during handling; wiping the bottom of the wells with lens paper prior to photographing may improve clarity. The assay may be conducted longer than 6 days if desired; however, changing cell culture medium may be required to maintain cell viability.
- Analyze images using image analysis software to measure changes in the area of the structures to determine the extent of 3-D culture spheroid expansion for each sample.
- Image Analysis
Note: Images may be analyzed using free software such as ImageJ (http://rsb.info.nih.gov/ij/); see below. Other image analysis software may be configured to make the same measurements; please consult your software supplier regarding capabilities and instructions.- Photograph image of known size (eg. 1 mm) using 4X objective, and measure pixels in ImageJ.
- Open image. Go to File/Open, and select image.
- Select line tool.
- Draw a line the length of the object.
- Go to Analyze/Measure, and record pixel number.
- Calculate the number of pixels in each mm (eg. 600 pixels/mm). Record result ------- pixels/mm
- Set scale; this will need to be done each time ImageJ is opened.
- Go to Analyze/Set Scale.
- For “Distance in pixels” input value from VIII.B.4.v. (above).
- For “Know distance” input “1,000”.
- For “Unit of Length” input “um”.
- Check “Global”, and select “OK”.
- Analyze spheroid image.
- Open image. Go to File/Open, and select image.
- Convert to an 8 bit image: Go to Image/Type/8 bit (check).
- Adjust image threshold. Go to Image/Adjust/Threshold (check). The program will distinguish between dark and light pixels, and the threshold may be adjusted on the histogram using the sliding scale. Similar settings will work for photographs taken under the same conditions. Areas of dark pixels will be overlaid with red. Adjust the threshold so that only the spheroid image is overlaid.
- Select spheroid. Select the circle tool and surround the image. This will eliminate any dark pixels outside of the spheroid from the measure.
- Go to Analyze/Measure. This will measure the area of the spheroid structure (μm2) in a table. Multiple measurements may be made in one table. Save table.
- Convert the table to an Excel worksheet. Open with Excel and “Save As” an .xls or .xlsx file.
- Photograph image of known size (eg. 1 mm) using 4X objective, and measure pixels in ImageJ.

Figure 2. Process for analysis of spheroid expansion. A) Capture image and convert to 8 bit; B) Set threshold to capture the total structure; C) Select structure to calculate total area.
- Fluorometric Analysis
- Add one tenth volume (10 μL per 100 μL) of Resazurin per well and transfer the plate back to the 37 °C cell culture incubator. See section VI.3 for reagent preparation.
- Read fluorescence at excitation 530-560 nm / emission 590 nm every hour between 1 and 4 hours. Optimal readings provide the largest dynamic range, best linearity, and least standard deviation.
- Graph data.
Data Interpretation
1. The CULTREX® 3-D Spheroid Fluorometric Proliferation/Viability Assay provides morphological and quantitative analysis of spheroid cell proliferation/viability. Spheroids expand in size as cells proliferate, and this results in changes in surface area that occur over time for proliferating cell lines (Figure 3). These changes in surface area can be measured and used to compare relative proliferation of different cell lines or cell lines subjected to genetic manipulation.
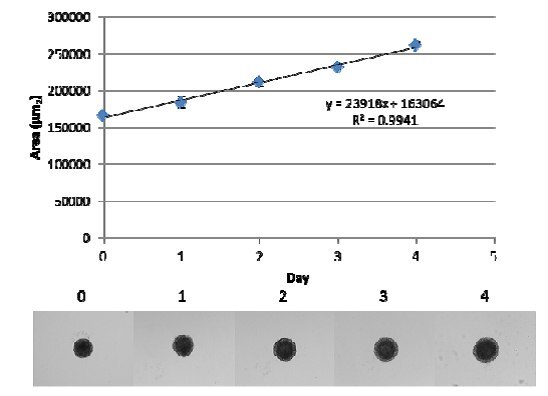
Figure 3. Spheroid growth of MDA-MB-231 breast cancer cells. Cells were seeded at 3,000 cells/well in the presence of spheroid formation ECM and incubated for 72 hours at 37 °C, 5% CO2 to induce spheroid formation. At that time, 50 µL of complete medium was added to each well, and spheroids were incubated at 37 °C, 5% CO2. Spheroids were photographed every 24 hours and images were analyzed using ImgaJ software.
- The changes in area can also be used to evaluate the effect of pharmacological compounds on spheroid growth. Once optimal assay conditions have been established, this can be evaluated as an endpoint assay. The following assay was conducted using MDA-MB-231 cells treated with varying concentrations of the inhibitor Bleomycin.
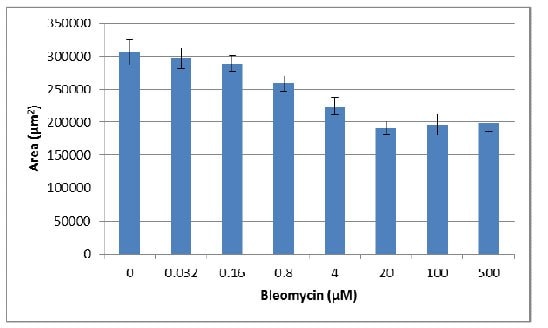
- Next the area for each spheroid prior to treatment was subtracted to determine spheroid expansion.
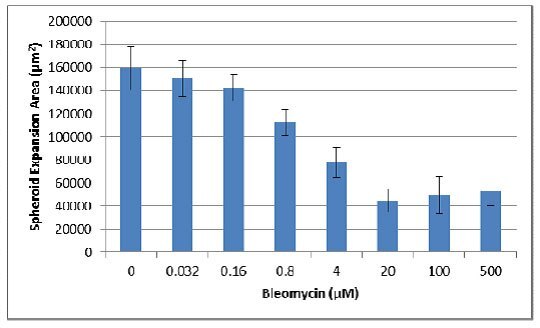
- Finally, the resulting areas were divide by the area obtained for the no inhibitor control, providing the proliferation index as a percentage of control:
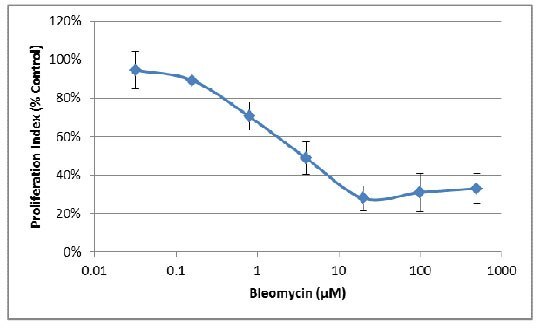
- Fluorometric endpoint analysis using Resazurin can be used to generate a standard curve based on cell seeding density. The following assay was conducted using MDA-MB-231 cells serially diluted from 3,000 cells/well to 94 cells/well on Day 0 and evaluated on Day 7.
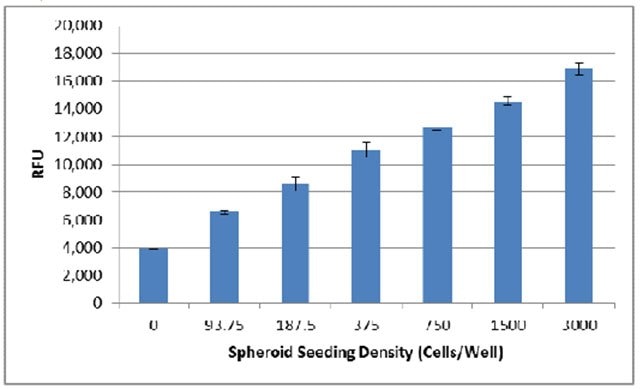
- Next the background was subtracted from each sample, and the data was plotted with a trendline to evaluate the relationship between cell seeding density and RFU:
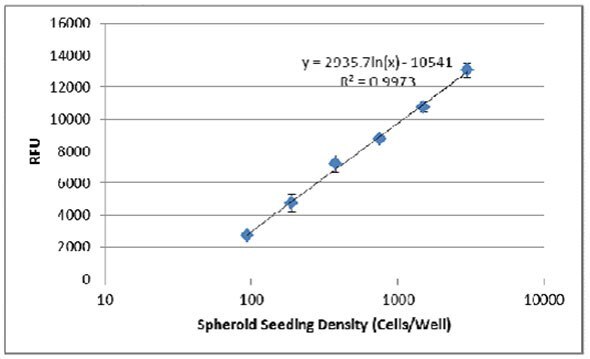
- Fluorometric endpoint analysis using Resazurin can also be used to evaluate the effect of pharmacological compounds on cell viability. The following assay was conducted using MDA-MB-231 cells treated with varying concentrations of the inhibitor Bleomycin.
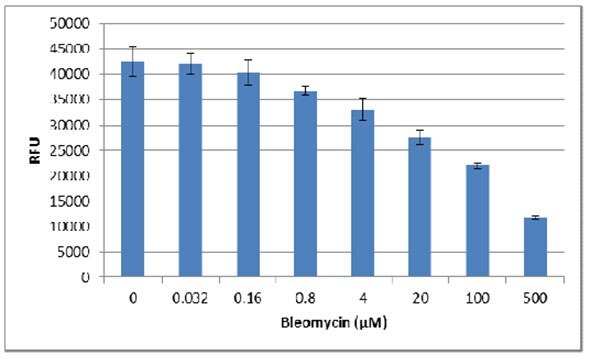
2. Next the background was subtracted from each sample:

3. Finally, the background-corrected RFUs were divided by the value obtained for the no inhibitor control, providing the viability index as a percentage of control:
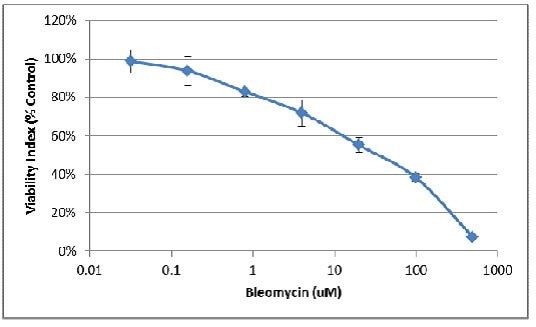
4. Using the linear portion of the inhibition curve, the IC50 may be estimated:
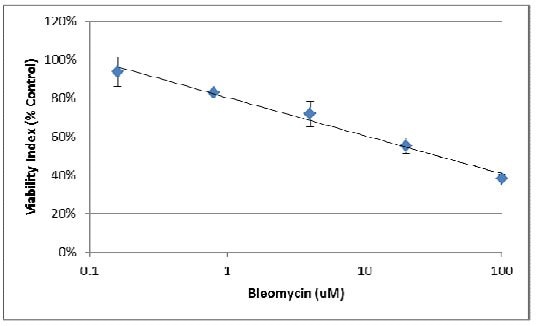
IC50 ~ 30 µM Bleomycin for MDA-MB-231 spheroids.
Troubleshooting Guide |
|---|
Legal Information
Cultrex is a registered trademark of Trevigen, Inc.
References
To continue reading please sign in or create an account.
Don't Have An Account?