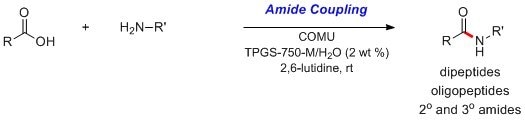
Amide Coupling in a Box
Product No. 802387
Reagents |
|---|
Precautions
Please consult the Safety Data Sheet for information regarding hazards and safe handling practices.
Procedure
- Add the amine (0.5 mmol) and the carboxylic acid (0.55 mmol) into the provided 5-mL reaction vial containing a stir bar and preloaded reagent COMU (240 mg, 0.56 mmol) under N2.
- Replace the solid top cap with the provided septum top cap and connect with N2 bubbler through a needle.
- Transfer the provided TPGS-750-M/H2O (1.0 mL; 2 wt %) solution and 2,6-lutidine (0.19 mL, 1.63 mmol) into the reaction vial using the provided syringe.
- Turn on the stirrer and stir the resulting mixture vigorously at room temperature until the reaction is complete (monitored by TLC, utilizing bromocresol green and ninhydrin or KMnO4 to indicate disappearance of carboxylic acid and amine starting materials, respectively).
- After completion, extract the reaction mixture with 3 x 2 mL of i-PrOAc (or EtOAc or MTBE) followed by wash the organic extracts with 2 x 6 mL of HCl (1 M) and 3 x 6 mL of Na2CO3 aqueous saturated solution.
- Pass the organic phase through a plug of silica and wash the silica gel bed with an additional 3 mL of i-PrOAc (or EtOAc or MTBE) and collect all of the coupled material.
- Remove all of the volatiles in vacuo and purify the crude material by flash column chromatography on silica gel.
Notes: Less polar extraction solvents such as MTBE or i-PrOAc are generally use in order to minimize the partition of the oxime into the organic phase during the base extractions. Additional base extractions may be required if EtOAc is used as extraction solvent. Most peptide coupling products can be isolated without additional purification; however, column chromatography can be utilized for further purification.
More information on the research from the Lipshutz Group can be found in the Professor Product Portal.
Additional information on TPGS–750–M: Second Generation Amphiphile for Organometallic Chemistry in Water at Room Temperature can be found in the Technology Spotlight section of our website.
Reference
Visit our protocol page
To continue reading please sign in or create an account.
Don't Have An Account?