Determination of Fatty Acid Content in Infant Formula by GC/FID Using the SP-2560 Capillary Column per AOAC Method 2012.13
Katherine K Stenerson, Olga I Shimelis, Gary Oshi
Reporter US, Volume 33.4 (Food & Beverage Supplement)
The nutritional content of infant formula is considered extremely critical, and thus it is one of the most regulated food products worldwide. Most commercial infant formulas are derived from cow’s milk, and are formulated to mimic human breast milk. They offer the advantage of being nutritionally balanced and easy for most babies to digest1. All infant formulas, whether cow’s milk-based or derived from another source, are subject to specific regulatory requirements regarding nutritional content.
With regards to fats, the U.S. Food and Drug Administration (FDA) requirements include an acceptable range for fat content, and a required minimum content for linoleic acid2. The European Union (EU) has specified minimum fat and detailed fatty acid composition requirements that include maximum allowable levels for trans as well as other fatty acids, and specific ranges and concentration levels for polyunsaturated fatty acids (PUFAs)3. In order to ensure that these requirements are met, manufacturers of infant formulas must conduct testing on their products to determine fatty acid content and composition.
In 2010, the Association of Official Analytical Chemists (AOAC) came to a consensus agreement with the International Formula Council (IFC) to establish standard method performance requirements for testing infant formula. Since many testing methods already existed, both compendial (such as AOAC, ISO, etc) and private (i.e. company specific), the need existed to establish fully validated reference methods which were globally recognized4.
Recently, AOAC adopted method 2012.13 which describes the analysis of fatty acid content using direct in-matrix transesterification followed by gas chromatography flame ionization detector (GC/FID) analysis on a highly polar cyanosilicone capillary column5. The GC column chemistry specified in this method allows for detailed characterization of the fatty acid composition in milk products and infant formula samples. In the work presented here, AOAC Method 2012.13 was applied to the analysis of powdered infant formula. Following the prescribed method, which included use of the 100 m SP™-2560 capillary column, resulted in identification and quantitation of all labeled fatty acids and fatty acid groups (such as trans fatty acids). The fatty acid groups determined using the method are described in Table 1.
Method
Sample preparation of powdered infant formula was done following the protocol outlined in AOAC method 2012.13 (Table 2). As required by the method, GC analysis was conducted on the 100 m SP-2560, which is a highly polar cyanopropyl silicone capillary column. The length, high polarity, and selectivity of this column are necessary to facilitate resolution of the cis and trans monounsaturated fatty acids (MUFAs) in the oleic (C18:1) region. The GC run conditions used are described in Figure 1.
Prior to analysis of samples, the method requires that system resolution be verified to ensure sufficient separation of the C18:1 cis/trans isomers. This was done by injecting the cis/trans FAME column performance mix (Product No. 40495-U). This is a qualitative mix that contains a mixture of C18:1 trans and C18:1 cis isomers. Resolution is calculated between two groups of FAME isomers. Group 1 = C18:1Δ13t and C18:1Δ14t, whereas group 2 = C18:1Δ9c (oleic acid) and C18:1Δ10c. A resolution value of >1.0 is considered acceptable. As shown in Figure 1, the resolution value calculated using the SP-2560 column was R = 1.12.
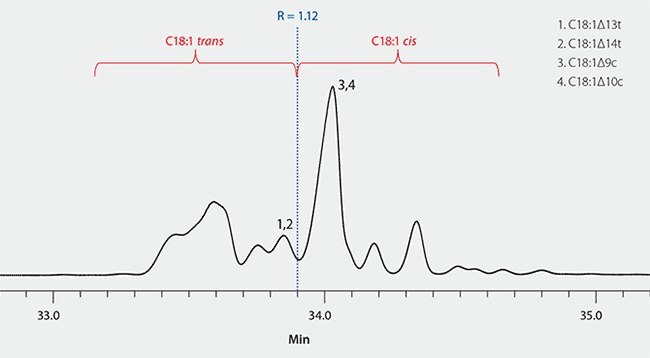
Figure 1.Resolution Check of SP-2560 using cis/trans FAME Column Performance Mix per AOAC Method 2012.13
GC Conditions
column: SP™-2560, 100 m × 0.25 mm I.D., 0.20 µM (Product No. 24056); oven: 60 °C (1 min), 15 °C/min to 165 °C (1 min), 2 °C/min to 225 °C (20 min); inj. temp.: 250 °C; carrier gas: helium, 0.8 mL/min; detector: FID, 250 °C; injection: 1 µL, 10:1 split; liner: 4 mm I.D., split/splitless type, wool packed single taper FocusLiner™ design; sample: cis/trans FAME Column Performance Mix (Product No. 40495-U)
The in-matrix transesterification approach described in the method greatly simplifies sample preparation. Since it results in the conversion of fatty acids present in acylglycerols to fatty acids methyl esters (FAMEs), the efficiency of this process is critical to obtaining accurate results for fatty acid content. Thus, the method requires that transesterification performance be monitored. This is done by addition of two internal standards to each sample; methyl undecanoate (C11:0 FAME) and glyceryl tritridecanoate (C13 TAG). The transesterification process generates C13:0 FAME from the C13 TAG. A stoichiometric conversion factor is then used to convert the response of the C13:0 FAME to the C13:0 TAG. The performance of the tranesterification process (Pt) is calculated as a percentage of C13:0 TAG to C11:0 FAME. The Pt value must fall within the range of 98-102% for the corresponding sample data to be acceptable. For our sample, the Pt was calculated to be 98.2%, indicating acceptable transesterification.
After analysis of the sample, the fatty acids are identified by retention time comparison to a standard containing C4:0 – C24:0 saturated fatty acids (SFAs), C15:1 – C20:1 monounsaturated fatty acids (MUFAs), and C18 – C22 polyunsaurated fatty acids (PUFAs). This same standard is used for calculation of response factors, which are in turn used to calculate the levels of each fatty acid identified in the sample.
Results
The chromatogram obtained from the powdered infant formula sample is shown in Figure 2 with the fatty acids identified. Matrix peaks were present which may have interfered with detection of some fatty acids; namely eluting in the regions of C20:3n6, C22:2n6 and C24:1n9. Some carryover from a previous run was observed eluting after 39 minutes. To prevent this, a final oven hold time of >20 minutes is recommended when using helium carrier gas.
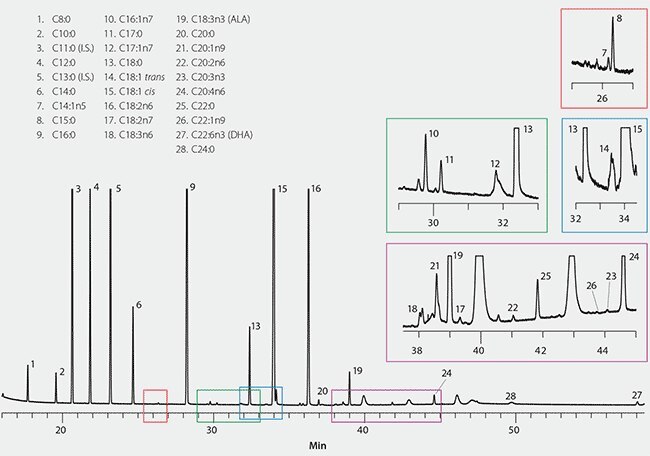
Figure 2.GC Analysis of Fatty Acids in Powdered Infant Formula on SP-2560
GC Conditions
column: SP™-2560, 100 m × 0.25 mm I.D., 0.20 µM (Product No. 24056); oven: 60 °C (1 min), 15 °C/min to 165 °C (1 min), 2 °C/min to 225 °C (20 min); inj. temp.: 250 °C; carrier gas: helium, 0.8 mL/min; detector: FID, 250 °C; injection: 1 µL, 10:1 split; liner: 4 mm I.D., split/splitless type, wool packed single taper FocusLiner™ design
Calculation of the fatty acid amounts present was done per the method, and the results are summarized in Table 3. The total fatty acid content is broken down by class, with unsaturated classes further speciated as omega 3, 6, and 9 fatty acids. Of the omega 3 fatty acids, the level of C18:3n3/a-linolenic acid (ALA) was the highest, followed by C22:6n3/docosahexaenoic acid (DHA).
Both of these are essential to body function and structure, and must be obtained through diet since they cannot be synthesized by the human body. Some ALA can be converted in the body to DHA, and this percentage is higher in infants than adults.6 The SP-2560 provided good resolution between the C18:1 cis and trans portions of the sample, thus allowing for accurate determination of the trans fatty acids (TFAs). Trans fats have been associated with an increased risk of coronary heart disease, and thus minimal dietary intake is recommended. They are produced during hydrogenation of vegetable oils, and occur naturally in the stomachs of ruminants such as cows. The TFA results obtained with this sample were extremely low, well below the EU limit (Table 4). In addition to TFA, results obtained for other key fatty acids were compared to EU and FDA requirements, as shown in Table 4. As is indicated, this sample was within the specified fatty acid content requirements for both sets of regulations.
Conclusions
Preparation and analysis of powdered infant baby formula per AOAC method 2012.13 provided for accurate determination of the fatty acid content. The sample preparation was simple and fast, and chromatographic analysis using the SP-2560 provided resolution of all key fatty acid methyl esters, including resolution between the C18:1 cis and trans positional isomers. The information determined with the method allowed for comparison of the sample to both EU and FDA regulations pertaining to fatty acid content in baby formula.
To continue reading please sign in or create an account.
Don't Have An Account?