Analysis of Residual Solvents in Hemp Extract by Headspace SPME-GC/MS
Katherine K. Stenerson
Reporter US Volume 34.2
A straightforward, fast, sensitive, and accurate alternative to headspace analyzers
Marijuana oil, produced by extraction of the flower buds of the cannabis sativa plant, has reputed therapeutic benefits.1,2 The extraction process typically involves solvents such as hexane or some alcohols that may remain in the final product.3 The residual solvents are typically measured using a headspace analyzer interfaced directly to a GC. Solid phase microextraction (SPME) is an alternative approach that is often faster and easier to use. The method outlined here uses SPME with GC/MS finish to measure solvents in hemp extract. Hemp and marijuana are both varieties of cannabis sativa, however hemp is a variety that is very low in THC content.
Experimental
Analytical conditions appear in Figure 1. The sample comprised 99% pure hemp extract in a base of hempseed oil, unspiked and spiked at 10 ng/g with the solvents listed in Table 1. Quantitation was by external standard against a 6-point curve from 6 to100 μg/g prepared in soybean oil. Blank soybean oil was analyzed along with SPME fiber blanks to verify cleanliness of the system prior to testing and that there was no carryover between standards and samples. A Carboxen® (CAR)/PDMS SPME fiber was chosen for the extraction step due to its ability to retain low molecular weight compounds with differing polarities, such as hexane and methanol. The column for the GC/ MS analysis, Supel™-Q PLOT, was chosen for its ability to retain very low molecular weight compounds, and for its selectivity toward a variety of compounds, including alcohols, aliphatics, and aromatics. Supel-Q PLOT also has the ability to retain lighter aliphatics, such as butane and pentane, and thus would be a suitable choice if these compounds are to be analyzed.
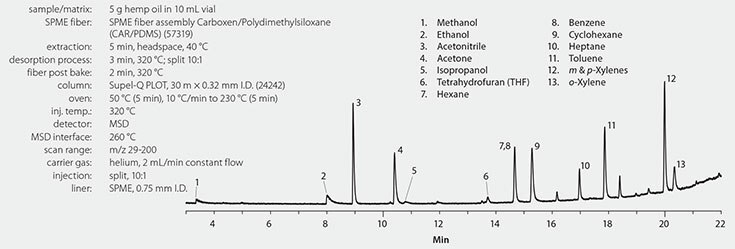
Figure 1.Hemp Extract Spiked at 10 ng/g with Solvents, Analyzed by SPME-GC/MS
*measured together
NA: not analyzed due to high level detected in unspiked sample
Solvents in classes I, II and III by the International Conference on Harmonization (ICH) guidelines.4
Results and Discussion
A representative chromatogram of a spiked hemp extract sample is shown in Figure 1. The coelution of benzene/cyclohexane was resolved using unique m/z values for quantitation of each compound. A summary of accuracy and precision for the analyses of n=3 replicates of hemp extract, spiked at 10 μg/g, is presented in Table 1. Hexane was present in the unspiked hemp extract at six times the spiking level and thus is not reported for the spiked samples. The hexane level reported for the unspiked extract is the average of duplicate analyses, with the percent reproducibility of the measurement (13%) shown in parentheses. For the remaining solvents in the analyte list, accuracy was >90% for all compounds except ethanol, which was 84%. Precision, determined as %RSD, was good with values of <10% for all spiked compounds.
The use of SPME allowed for the straightforward, fast, sensitive, and accurate GC/MS determination of the residual solvent content of spiked and unspiked samples of hemp extract. The CAR/PDMS SPME fiber in combination with the Supel-Q PLOT GC column provided the selectivity for the solvents analyzed, and could be used for lists including lighter compounds, such as butane and propane.
References
To continue reading please sign in or create an account.
Don't Have An Account?