FSL Construct Protocol
Adapted from Blake, D.A., et al. FSL Constructs: A simple method for Modifying Cell/Virion Surfaces with a Range of Biological Markers Without Affecting their Viability. J. Vis. Exp. (54), e3289, doi:10.3791/3289 (2011).
The following protocol describes the generic procedure for the insertion of FSL constructs (Figure 1) into biological membranes. For simplicity the protocol will only refer to cells (kodecytes), which are at a 100% concentration. However, the term cells is interchangeable with virions (kodevirions) or organisms or cellular structures and their concentration in diluent is not important, provided it is always consistent between tests, controls, and experiments. With red blood cells the packed cell volume is typically 80%, but with virions, embryos and other cells it is typically less than 1%. The method is very robust and will produce modified membranes when used by any of its variations, but refinement will be required to optimize and standardize the degree of modification required for specific applications.
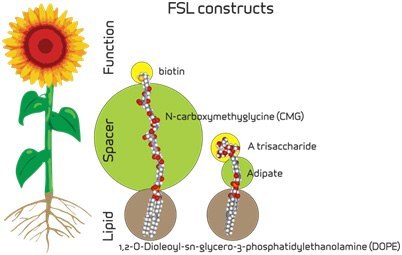
Figure 1.
Preparation of FSL Constructs
- To prepare the FSL construct Stock Solution, first reconstitute the dry FSL product by the addition of diluent to the product vial as specified in Table 1. Briefly sonicate (30 seconds). This will prepare a Stock Solution with concentration as indicated in Table 1. Aliquot into 100 μL sterile containers and stored at 2-8 °C for up to one week, or frozen for up to 3 months.
- To prepare FSL construct Working Solution for insertion, just prior to use briefly sonicate (30 seconds) the Stock Solution to homogenize any micelles. Dilute the FSL construct Stock Solution in buffer (preferably not containing lipids or highly hydrophobic material) to the concentration required or over a range if desired. FSL construct Working Solutions should be stored at 2-8°C and used within a few days.
Notes:
- FSL constructs will usually insert into cells in lipid containing media but will typically require as much as a 50X higher FSL working concentrations than if in PBS or other lipid free media.
- The working range dilutions will depend on the application, the detection method sensitivity, the type of FSL construct, and the degree of modification required. Typically the range of dilution will be between 10-500 μg of FSL per mL of diluent for carbohydrate FSL constructs and 1-100 μg/mL for other FSL constructs such as fluorophores and biotin.
- If preparing insertion diluents containing multiple FSL construct, simply add the constructs together in the same diluent at their normal working concentration. If one construct is at much higher concentration than another, some adjustment (increase) in concentration may be required for the lesser construct. Alternatively constructs may be added sequentially, preferably with the highest concentration construct first, followed by the lesser.
- FSL construct concentrations above 1000 μg/mL may introduce significant amounts of lipid into a cell membrane, and may change the shape of the cell or make it more susceptible to lysis.
- FSL constructs can be diluted in water but will have reduced stability and must be used within hours. If the package insert specifies water as the reconstitution diluent the product in the vial will also contain salts (as specified in the package insert).
Insertion of FSL Construct(s) into Membranes
- First wash the cells for FSL modification free of unbound lipids by centrifugation and using PBS or lipid free cell media as wash solution.
- Pack the cells or suspend in 100 µL of diluent.
- Simultaneously prepare controls which ideally should be both unmodified cells (incubated with PBS rather than FSL solution) and/or cells modified with a benign but related FSL construct.
- Add 100 μL of an appropriate dilution of FSL solution (containing 1 or more FSL constructs) to the cells and incubate for 1-2 hours at 37 °C. A similar result can be obtained by 6 hours incubation at 25 °C or overnight (18 hours) at 4 °C – mixing is recommended every few hours if heavy cell suspensions are used.
- Wash (optional) twice with PBS or media to remove any free FSL constructs and prepare an appropriate suspension.
- Once the FSL modification process has been completed, the kodecytes can be stored at 4-8 °C in lipid free media or used immediately.
Notes:
- The diluent used to suspend the cells can be cell culture media, PBS, cell storage solutions, etc, but preferably without lipids (e.g. fetal calf serum) or detergents (e.g. TWEEN).
- Different volumes (than 100 μL), ratios (than 1:1 cells/FSL solution) or incubation conditions of time and temperature may be used provided the same ratio, concentration, and volume are used to obtain reproducible results.
- The rate of FSL insertion is primarily determined by FSL concentration, incubation time, and temperature.
Handling and Visualization
- Kodecytes and kodevirions generally behave the same as unmodified cells/virions and can be handled and viewed under normal testing systems (microscopy, serology, flow cytometry, etc). They usually have no special requirements except for the avoidance of solvents/detergents/lipids that may elute the lipid constructs from membranes.
Notes:
- Constructs will remain in the membrane of inactive cells, such as red cells, if stored in lipid free media for the life of the cell. In cells with active membranes the constructs will be internalized and metabolized, at a rate dependent on cell membrane activity.
- Dead cells will usually contain high levels of FSL constructs; this can be used to separate nonviable cells from viable cells.
- If kodecytes are stored (or used in vivo) in lipid containing media/environments, such as serum/plasma the construct will slowly elute from the membrane over a period of hours or days, depending on temperature and lipid concentrations/compositions.
References
To continue reading please sign in or create an account.
Don't Have An Account?