A Validated Amino Acid Analysis Assay for Accurate Quantification of Stable Isotope Labeled Protein Reference Materials: SIL Thyroglobulin Case Study
Kevin Ray, Pegah, R, Jalili, Mark Angeles, James, J, Walters, Isil Dilek, and Uma Sreenivasan
Overview
With the emergence of mass spectrometry for the clinical measurement of proteins in biological matrices, the development of biological reference materials will continue to grow in importance. Certified Reference Materials with values assigned by metrologically valid procedures will be critical to minimize and control experimental variations in all steps of the workflow including protein extraction, fractionation, enrichment, proteolysis and analysis.
We have produced a stable isotope labeled (SIL) full length Thyroglobulin for use as an internal standard in quantitative MS workflows and have verified sequence fidelity and isotopic incorporation.
We have also developed an amino acid analysis (AAA) method traceable to NIST SRM 2389 and validated the method against NIST SRM 927. This AAA method will be used for accurate quantification of proteins to enable development of accuracy-based protein reference materials.
Characterization of SIL-Thyroglobulin |
|---|
AAA Workflow Overview

Figure 1.Control BSA is prepared by gravimetrically dilution of SRM 927. Tg samples are gravimetrically diluted to ~150 μg/mL. All controls and samples are gravimetrically dispensed into hydrolysis tubes. After internal standard is added, samples are hydrolyzed, labeled and analyzed by LC-UV (Waters AccQ-Fluor Kit, Prod. No. WAT052880). Calibration standards are gravimetrically prepared from SRM 2389.
Sequence Verification of SIL-Thyroglobulin

Figure 2.Composite sequence coverage of SIL-Thyroglobulin from peptide mapping with four proteolytic enzymes after deglycosylation with PNGase F (Prod. No. P7367) was >92%. Proteolytic enzymes used were Trypsin (Prod. No. T6567), GluC, AspN (Prod. No. P3303), and Chymotrypsin. After enzymatic digestion, SIL-Thyroglobulin samples were analyzed via RP LC-MS/MS using a Waters Acquity M Class, coupled to a Waters XEVO-G2S QTOF. A Waters BEH130 C18 100mm x 100 uM x 1.7 uM column was used, with the following LC conditions: flow rate of 750 nL/min, mobile phase A of 0.1% formic acid (Prod. No. 56302) in water, mobile phase B of acetonitrile (Prod. No. 34967), and gradient of 1% mobile phase B at start, 1-40% mobile phase B in 45 minutes, 40-90% mobile phase B in 47 minutes, 90% mobile phase B at 49 minutes, 90-1% mobile phase B in 51 minutes, and ended at 1% mobile phase B at 80 minutes.
Isotopic Incorporation of SIL-Thyroglobulin

Figure 3.Incorporation of 13C6 15N4 labeled arginine in two surrogate peptides liberated from SIL-Thyroglobulin was >98%. SIL-Thyroglobulin was first reduced in a solution of Tris(2-carboxyethyl)phosphine hyrdochloride (Prod. No. C4706), then alkylated with Iodoacetamide (Prod. No. A3221), before digestion with trypsin (Prod. No. T6567). After enzymatic digestion, SIL-Thyroglobulin was analyzed via RP LC-MS/MS using a Waters Acquity M Class, coupled to a Waters XEVO-G2S QTOF. Two Supelco BIOshell A160 Peptide C18 15 cm x 300 µM x 2.7 µM columns (Prod. No. 67093-U) were run in tandem, with the following LC conditions: flow rate of 10 µL/min, mobile phase A of 0.1% formic acid (Prod. No. 56302) in water, mobile phase B of acetonitrile (Prod. No. 34967), and gradient of 3-45% mobile phase B in 60 minutes, 45-90% mobile phase B in 61 minutes (held for two minutes), and 90-3% mobile phase B in 64 minutes.
Purity Confirmation of SIL-Thyroglobulin
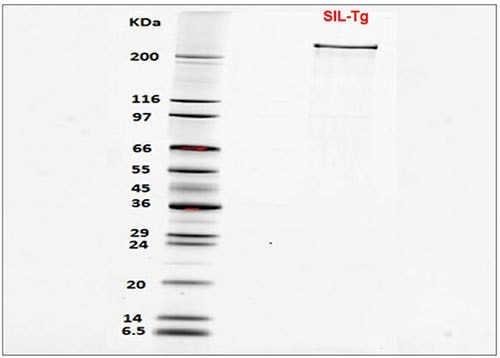
Figure 4.SDS-PAGE of SIL-Thyroglobulin showing purity >95%. Samples were first reduced using 2X Laemmli Sample Buffer (Prod. No. S3401). 250 ng sample was then loaded on gel, along with molecular weight marker SigmaMarker (Prod. No. S8445). After electrophoresis, gel was stained using SYPRO Ruby gel stain (Prod. No. S4942) before visualization.
AAA Assay Figures of Merit |
|---|
BSA-SRM 427 Value Assignments
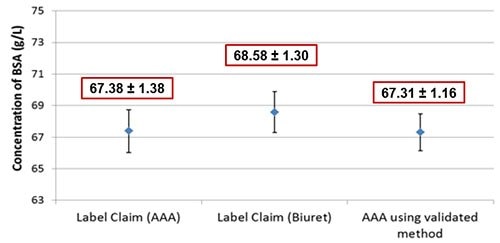
Figure 5.NIST SRM 927 was measured using AAA as described in Figure 1. The measured mean using AAA falls within the expanded uncertainty of SRM 927 as reported by NIST. Mean ± expanded uncertainty for 95% confidence are shown.
Tg - CRM 457 Value Assignments
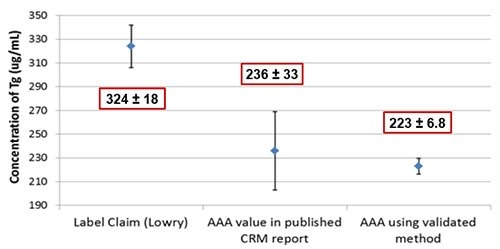
Figure 6.Tg CRM 457 was measured using AAA as described in Figure 1. The measured mean falls within the expanded uncertainty as reported by BCR for AAA1, but is much lower (~30%) than that of the label claim as measured by Lowry assay. Mean ± expanded uncertainty for 95% confidence are shown.
Summary
- We have validated an amino acid analysis (AAA) method traceable to NIST SRM 2389 to enable accuracy-based value assignment of protein reference materials.
- SIL-Tg characterization demonstrated >92% sequence coverage, isotope incorporation of >98%, and purity >95% by SDS-PAGE.
To continue reading please sign in or create an account.
Don't Have An Account?