Development of Simple and Rapid Workflows for Quantitation of Infliximab and Adalimumab in Human Serum by LC-MS/MS
Kevin Ray, Yue Lu, Pegah R. Jalili
Overview
There is a growing demand for reliable LC-MS/MS assays to support quantitation of serum levels of infliximab and adalimumab monoclonal antibodies, as clinical responses differ between patients due to varying pharmacokinetics and the formation of autoantibodies. We have optimized a simple “pellet digestion” sample preparation workflow that can be completed for 96 samples in less than 4 hours. We have also evaluated the optimized “pellet digestion workflow” in a Control Flow Plate (CFP) format.
Methods
Human serum samples were fortified with infliximab and adalimumab at concentrations ranging from 1.2 to 312.5 μg/mL. Samples were then spiked with 20 μg/mL stable isotope labeled versions of each antibody. To precipitate the protein, ice-cold methanol was added for plate digestion while ice-cold acetonitrile was added for control flow plate digestion. The resulting protein pellet was digested with trypsin for 2 hours at 600 ◦C. A manual protocol requiring centrifugation was compared to an automated protocol using Tecan Control Flow Plates. Tryptic peptides were separated on a Supelco BIOshell A160 Peptide C18, 2.7 μm fused core particle column; 10 cm x 500 μm. Detection was performed in MRM mode on Sciex QTRAP 5500 system.
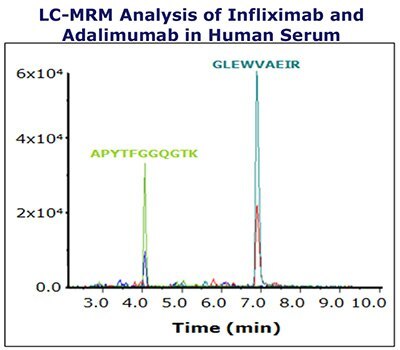
Figure 1. Extracted ion chromatogram (XIC) of one unique Infliximab peptide and one unique Adalimumab peptide.
Pellet Digestion Workflow

Figure 2.Pellet Digestion Workflow
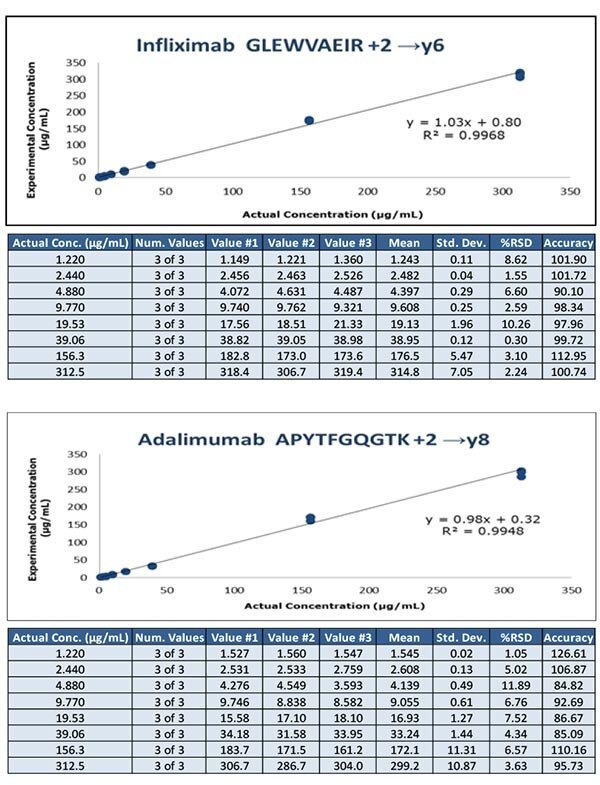
Figure 3. Calibration curves obtained from human serum containing 1.2 – 312.5 μg/mL of Infliximab and Adalimumab spiked with 20 μg/mL SILuMab internal standards using pellet digestion workflow.
Pellet Digestion Workflow Summary
- A lower limit of quantitation of 1.2 μg/mL for adalimumab and infliximab from 20 μL of serum was achieved with the optimized pellet digestion workflow. This protocol is sensitive, but requires centrifugation and manual processing steps.
Control Flow Plate Workflow

Figure 4. Control Flow Plate workflow
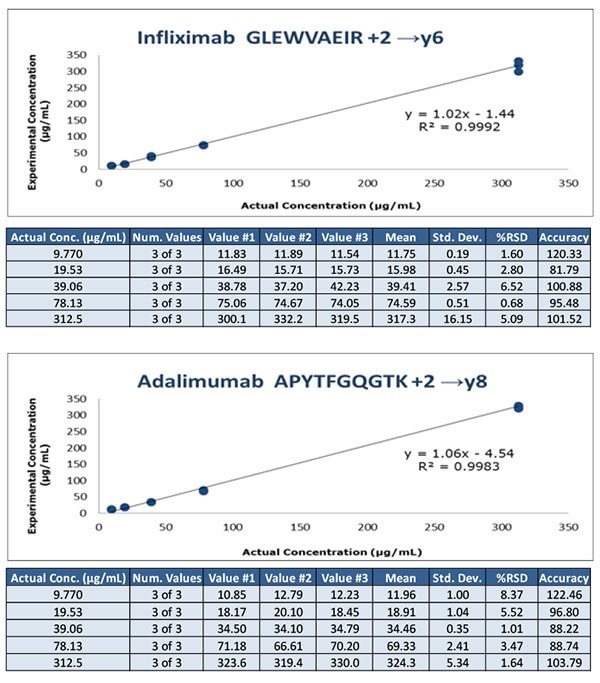
Figure 5. Calibration curves obtained from human serum containing 9.8 – 312.5 μg/mL of infliximab and adalimumab spiked with 20 μg/mL SILuMab internal standards using CFP workflow.
Control Flow Plate Workflow Summary
- The CFP protocol provides greater automation potential by eliminating manual processing steps, but the CFP protocol used here is less sensitive, with lower limit of quantitation of 9.8 μg/mL for infliximab and adalimumab. Further optimization of the CFP protocol is required for better sensitivity.
To continue reading please sign in or create an account.
Don't Have An Account?