Parabens in Topical Preparations Using SPME-GC-MS
Katherine K. Stenerson
Reporter US Vol 25.4
Introduction
Parabens are commonly used preservatives found in many commercial products, such as pharmaceutical and cosmetic topical preparations. While these compounds are generally considered safe, there is growing concern due to their detection in breast tumor tissue (1).
Due to the sample matrices (creams, lotions, and ointments) that must be investigated, sample preparation tends to be complex. The use of solid phase microextraction (SPME) as a simpler sample preparation / sample introduction technique for parabens in these matrices, prior to analysis via ion mobility spectrometry (IMS) has been demonstrated (2).
In this article, the use of SPME in conjunction with capillary gas chromatography-mass spectrometry (GCMS), a more common analytical technique than IMS, for the analysis of parabens was investigated. While GC analysis times cannot approach the quickness obtained with IMS, GC has the advantage of being available in many laboratories.
Experimental
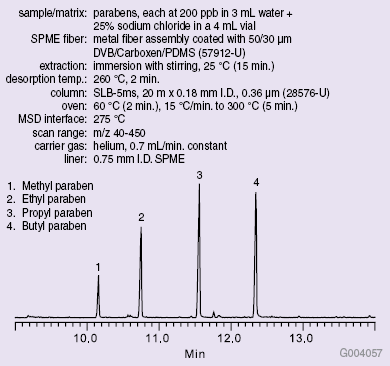
A series of six calibration standards ranging in concentrations from 25 to 300 μg/L of each analyte were prepared. SPME was used to extract the parabens from each standard prior to GC-MS analysis on an SLB™-5ms capillary GC column. Conditions used and the resulting chromatogram of the 200 μg/L standard are shown in Figure 1.
SPME-GC-MS was then used with three ‘real-world’ samples to confirm its applicability for complex matrices. These included a paraben-free ointment spiked with parabens, a paraben-containing arthritis lotion, and a paraben-containing anti-itch cream.
Results
Calibration.
All R2 values from the calibration were in the range from 0.9811 to 0.995, indicating good linearity. These calibration curves were subsequently used to determine values for the three ‘real-world’ samples analyzed as part of this work.
Spiked ointment.
A paraben-free betamethasone valerate ointment was spiked with each paraben then processed. Each analyte was well resolved from other components in the ointment. Percent recoveries ranging between 79 and 109 were obtained.
Arthritis lotion and anti-itch cream.
SPME-GC-MS results from both a paraben-containing arthritis lotion and a paraben-containing anti-itch cream were compared to results obtained from traditional high pressure liquid chromatography (HPLC) analyses. The results obtained by SPME-GC-MS were comparable to those obtained by traditional HPLC.
Discussion
The use of GC has distinct benefits over HPLC for complex matrices, namely due to the non-target components in the sample. With GC, simple sample preparation, such as SPME, can be used. As the inlet liner / head of the column become contaminated, they can easily be replaced / clipped. With HPLC, the column must be replaced when the front of the packing material in the column becomes contaminated. Therefore, more rigorous and timeconsuming sample preparation may be necessary to adequately remove non-target compounds from complex matrices prior to HPLC analyses.
Conclusion
In this article, it has been shown that the determination of parabens in topical products can be successfully performed using SPME-GC-MS. SPME is a simple and effective sample preparation / sample introduction technique. The SLB-5ms capillary column is an excellent choice due to its low bleed characteristics (up to 360 °C), highly inert nature, and impressive durability.
References
To continue reading please sign in or create an account.
Don't Have An Account?