Cultrex® 3-D Culture Matrix™ Basement Membrane Extract Coated 96 Well Plate Protocol
Product Number 3445-096-CP
I. Product Description
3-D Culture is an innovative approach to modeling the morphological effects of oncogenesis and development using three-dimensional microenvironments. Extracellular matrix proteins form hydrogels under physiological conditions that mimic the cell environment in vivo, and as a result, these cells assume structural and functional characteristics of their emanating tissues. The 3-D Culture Matrix BME Coated 96 Well Plate is coated with a reconstituted basement membrane matrix to provide the environment that is common for epithelial or endothelial cell types, and this plate provides a convenient, standardized, physiologically predictive format for evaluating the pharmacological effects of compounds for these cell culture models.
II. Specifications
- Source: Murine Engelbreth-Holm-Swarm (EHS) tumor
- Storage buffer: Dulbecco’s Modified Eagle’s medium without phenol red (Product No. D5030), with 10 μg/ml gentamicin sulfate (Product No. G1264)
III. Precautions and Limitations
- For Research Use Only. Not for use in diagnostic procedures.
- The physical, chemical, and toxicological properties of these products may not yet have been fully investigated; therefore, we recommend the use of gloves, lab coats, and eye protection while using these chemical reagents.
IV. Material Qualification
- Functional Assays
- Tube assay – Basement membrane extract promotes differentiation of a mouse endothelial cell line derived from axillary lymph node (SVEC4-10) into capillary – like structures.
- 3D culture – Basement Membrane Extract promotes differentiation of a human epithelial cell line derived from mammary gland (MCF-10A) or human prostate (PC-3) into acinar structures.
- Sterility Testing
- No bacterial or fungal growth detected after incubation at 37 ⁰C for 14 days following USP XXIV Chapter 71 sterility test.
- No mycoplasma contamination detected by PCR.
- Endotoxin concentration ≤20 EU/ml by LAL assay
- Gelling Assay
- BME gels in less than 30 minutes at 37 ⁰C, and maintains the gelled form in culture medium for a minimum of 14 days at 37 ⁰C.
V. Storage and Stability
Product is stable for a minimum of 3 months from date of shipment when stored at –20 ⁰C in a manual defrost freezer. For optimal stability, store at –80 ⁰C. Avoid freeze-thaw cycles.
VI. Culture Protocol
This procedure must be conducted in an aseptic environment, such as a laminar flow hood or clean room, using aseptic technique to prevent contamination.
- Culture cells as recommended by cell supplier to establish a stable population at 37 ⁰C in a CO2 incubator; growth media, growth factors, serum requirements, and incubation period may vary by cell type.
- Thaw 3-D Culture Matrix BME Coated 96 Well Plate at Room Temperature for one hour, and then transfer to a 37 ⁰C cell culture incubator for 30 minutes.
- Harvest cells from culture, and dilute cells to as needed in 3-D Culture Medium. Optimal cell seeding concentrations are cell line dependent and may need to be determined empirically. Also, please contact the cell supplier or consult the literature to determine what growth factors, cytokines, or other ECM proteins may be needed to support your cell model. Cells may also be treated with compounds during seeding if assessing the impact on proliferation.
- Add 100 μl of cell suspension to each well of the 96 well plate containing 3-D Culture Matrix BME.
- Incubate plate at 37 ⁰C in a CO2 incubator, and visually monitor cell growth and morphology. If assessing viability, cells may be treated once physiological structures are formed.
Recommendations for analysis:
Cell number may be determined using 3D Culture Proliferation Core Kit (cat# 3445-096-CK).
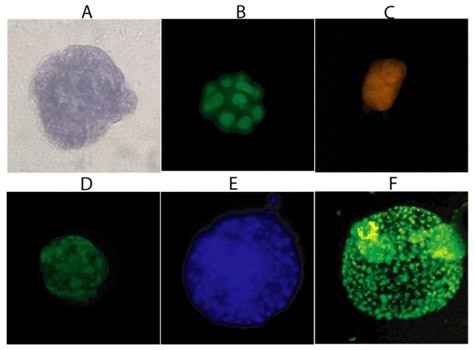
Figure 1.Three-Dimensional Cellular Structures. Staining of MCF-10A cells after sixteen days is 3-D Culture Matrix™ RGF BME with: A) Cell Staining Kit (structural), B) SYBR® Green (nuclear), and C) MitoShift™ (mitochondrial potential); and staining of PC-3 cells after twelve days in3-D Culture Matrix™ RGF BME with: D) Calcein AM (cell viability), E) CPA dye 1 (nuclear) and F) Depsipher™ (mitochondrial potential)
Legal Information
3-D Culture Matrix is a trademark of Trevigen, Inc.
Cultrex is a registered trademark of Trevigen, Inc.
References
Note: This product is made and marketed under patent license from the United States Public Health Service. Ref. U.S. Patent 4,829,000 issued May 9, 1989 and U.S. Patent 5,158,874 issued October 27,1992, all entitled Reconstituted Membrane Complex with Biological Activity.
To continue reading please sign in or create an account.
Don't Have An Account?