Trypsin Serine Protease Enzyme
What is Trypsin?
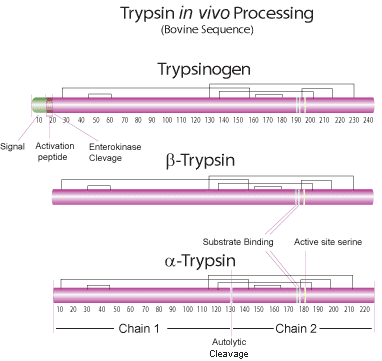
Trypsin is a member of the serine protease S1 family. It consists of a single chain polypeptide of 223 amino acid residues. The native form of trypsin is refered to as β-trypsin. Autolysis of β-trypsin (which is cleaved at Lys131- Ser132 in the bovine sequence) results in α-trypsin which is held together by disulfide bridges. The active site amino acid residues of trypsin include His46 and Ser183.2-5
What is Trypsin Used For?
Trypsin is often used for dissociation of adherent cells from the vessel in which they are being cultured, which is referred to as trypsinization. When added to a cell culture, trypsin breaks down the proteins that enable the cells to adhere to the surface. Upon completion of the trypsinization process, the cells will be in suspension and appear rounded.
Trypsin is also used to digest proteins for protein characterization and identification by mass spectrometry (MS). The process for protein digestion is performed in solution or in gel pieces sectioned from protein samples separated by gel electrophoresis. In-gel digestion of both silver and Coomassie-stained protein spots or bands and can be followed by matrix-assisted laser desorption/ionization (MALDI)-MS or liquid chromatography (LC)-MS/MS analysis to identify proteins at high sensitivities.
Physical Properties of Trypsin
In Vivo Processing
Trypsinogen, the proenzyme (zymogen) form of trypsin, is produced in the acinar exocrine cells of the pancreas. Three isoforms are excreted from the human pancreas. The cationic and anionic forms are the predominant human isoforms. The inhibitor-resistant mesotrypsinogen is found only in trace amounts.6 The proenzyme is activated only after it reaches the lumen of the small intestine. Enterokinase activates pancreatic trypsinogen to trypsin by the hydrolysis of a hexapeptide(for bovine trypsin at the Lys6 - Ile7 peptide bond) from the NH2 terminus. Bovine trypsinogen consists of a single polypeptide chain of 229 amino acids and is cross linked by six disulfide bridges. Trypsin can autocatalytically activate more trypsinogen to trypsin.
Trypsin Specificity, Kinetics, Substrates and Assay Methods
Specificity and Kinetics
Trypsin will cleave peptides on the C-terminal side of lysine and arginine amino acid residues. The rate of hydrolysis is slower if an acidic residue is on either side of the cleavage site and no cleavage occurs if a proline residue is on the carboxyl side of the cleavage site.
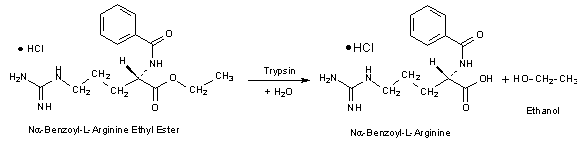
Trypsin will hydrolyze ester and amide linkages of several synthetic substrates: 2,7,8
Additional Substrates
The pH optimum of trypsin is 7 - 9.10
Assay Method11
The activity of most preparations is determined by a continuous rate spectrophotometric assay and expressed in BAEE units.
Unit Definition: One BAEE unit will produce a ΔA253 of 0.001 per min at pH 7.6 at 25 °C using BAEE as substrate. Reaction volume = 3.2 mL (1 cm light path).
Conditions
Temperature: 25 °C
pH 7.6, A253nm
Light path: 1 cm
In a 3.2 mL reaction mix, the final concentrations are 63 mM sodium phosphate, 0.23 mM Nα-benzoyl-L-arginine ethyl ester, 0.06 mM hydrochloric acid, and 100 units trypsin.
Reagents
- 67 mM Sodium Phosphate Buffer, pH 7.6 at 25 °C (Prepare 100 ml in deionized water using Sodium Phosphate, Monobasic, Anhydrous, Prod. No. S0751. Adjust to pH 7.6 at 25 °C with 1 M NaOH.)
- 0.25 mM Na-Benzoyl-L-Arginine Ethyl Ester Solution (BAEE) (Prepare 50 ml in Reagent A using Nα-Benzoyl-L-Arginine Ethyl Ester, Hydrochloride, Prod. No. B4500.)
- 1 mM Hydrochloric Acid Solution (HCl) (Prepare 50 ml in deionized water using concentrated Hydrochloric Acid, Prod. No. 258148.)
- Trypsin Enzyme Solution (Immediately before use, prepare a solution containing 500 BAEE units/ml of Trypsin in cold Reagent C.)
Procedure
Pipette (in milliliters) the following reagents into suitable quartz cuvettes:
Equilibrate to 25 °C. Monitor the A253nm until constant, using a suitably thermostatted spectrophotometer. Then add:
Immediately mix by inversion and record the increase in A253nm for approximately 5 minutes. Obtain the ΔA253nm/minute using the maximum linear rate for both the Test and Blank.
Calculation
df = Dilution factor
0.001 = The change in A253nm/minute per unit of Trypsin at pH 7.6 at 25 °C in a 3.2 ml reaction mix
0.20 = Volume (in milliliters) of enzyme used
Notes
- This assay procedure is not to be used to assay immobilized trypsins
- For the USP/NF procedure refer to the USP monograph
- This procedure is for informational purposes. For a current copy of our quality control procedure, please contact our Technical Service Department.
Unit Conversions
1 BAEE µM Unit = 200 BAEE Units
1 TAME µM Unit = 0.27 BAEE µM Units
1 BAEE µM Unit = 3.64 TAME Units
1 TAME µM Unit = 55 BAEE A253 Units
1 BAEE A253 Unit = 0.018 TAME µM Unit
1 TAME µM Unit = 180 TAME A247 Units
1 TAME A247 Unit = 0.33 BAEE Units
1 USP Unit = 3.0 BAEE Units
1 NF Unit = 1.1 USP Units
Trypsin Inhibitors
Serine protease inhibitors that inhibit trypsin include the following:2,11
Reconstitution and Solution Stability for Trypsin
Trypsin solutions in 1 mM HCl (pH 3) are stable for approximately 1 year when aliquoted and stored at -20 °C. The presence of Ca2+(20 mM) will also retard trypsin's ability to selfdigest itself (autolysis) and will maintain the stability of the trypsin in solution.2,9 Trypsin retains most of its activity in 2.0 M urea, 2.0 M guanidine HCl, or 0.1% (w/v) SDS.13 Trypsin is reversibly denatured at high pH (above 11), by precipitation with TCA, or by high concentrations of urea (greater than 6.5 M).3 In order to abolish all trypsin activity, heating at 100 °C in 1% (w/v) SDS for 5 minutes is required.14
Trypsinogen solutions are stable in acidic buffers (pH 2 - 4), while in neutral buffers the autocatalytic activation to trypsin occurs.
Trypsin Applications and Protocols
Cell Dissociation
Mitochondria Isolation
Protein Sequencing
Products
Trypsin for General Research Applications
Bovine Trypsin
Porcine Trypsin
Human Trypsin
Trypsin from human pancreas, salt-free, lyophilized powder, vial of ≥1,000 BAEE units
Trypzean™ Recombinant Bovine Trypsin, Expressed in Corn
TrypZean Powder
TrypZean Solution
Protein Sequencing
Trypsin Products for Proteomic/Protein Sequencing Analysis
Trypsin Solutions for Cell Dissociation
References
To continue reading please sign in or create an account.
Don't Have An Account?