Dinuclear Zinc Catalysts
The aldol reaction is arguably one of the most important C–C bond forming reactions in the synthesis of complex molecules. While the classical version is highly atom economical, it suffers from chemo- and regioselectivity problems. In the case of more selective, contemporary versions, nearly all of the reactions require pre-formation of an enolate, enol, or an equivalent thereof (e.g., silyl enol ethers in the Mukaiyama protocol). Generation of these species requires stoichiometric amounts of an adjunct reagent, decreasing from the overall atom efficiency of the process. Barry Trost and co-workers at Stanford University have developed a powerful catalyst technology that eliminates the requirement for pre-formation of a nucleophilic species. Moreover, aldol condensations, and variants thereof, can be performed in a highly asymmetric fashion. The catalyst system utilizes a proline-derived ligand in combination with Et2Zn (2 equivalents) to generate an active dinuclear zinc species in situ (Scheme 1).
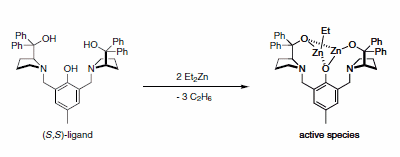
Scheme 1.Active dinuclear zinc species
The first demonstration of this catalyst was in the condensation of aryl methyl ketones with aldehydes.1 For example, the aldol product between isobutyraldehyde and acetophenone was obtained in good yield and in excellent optical purity (Scheme 2).
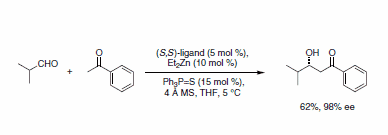
Scheme 2.Aryl methyl ketones
In a similar fashion, use of acetone as the nucleophilic aldol component provides access to a variety of chiral b-hydroxyacetones (Scheme 3).2
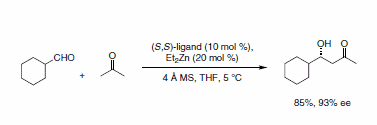
Scheme 3.Chiral b-hydroxyacetones
Trost’s catalytic asymmetric aldol reaction is applicable to α-hydroxyketone donors, giving synthetically useful polyoxygenated products in a stereocontrolled fashion. The reactions proceed at room temperature in high-yield with good diastereoselectivity in favor of the syn-diol adduct.3 This methodology was utilized in the synthesis of the natural product (+)-boronolide (Scheme 4).4

Scheme 4.Natural product (+)-boronolide
Similarly, α-hydroxyketones undergo imine addition in the presence of the dinuclear zinc catalyst. This Mannich-type reaction is highly diastereo- and enantioselective, giving syn-1,2-amino alcohols in very good yield.5 Both glyoxalate imines and aldimines are active substrates in the imine aldol reaction (Scheme 5).
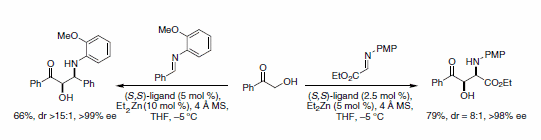
Scheme 5.Imine aldol reaction
Despite tremendous potential, the instability of methyl vinyl ketone and its aldol adducts has hampered its use in synthesis. Recently, the Trost group reported the general catalytic asymmetric aldol reaction using methyl vinyl ketone in combination with the dinuclear zinc catalyst (Scheme 6).6
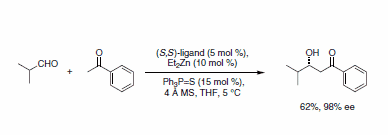
Scheme 6.Dinuclear zinc catalyst
Until the recent development of the zinc catalyst system, the use of methyl ynones in aldol additions was also virtually unknown. Addition of methyl ynones to α-ketal aldehydes occurs smoothly to give highly functionalized alkyne building blocks (Scheme 7).7 This strategy was used to install the C9 stereochemistry in the formal synthesis of the cytotoxic phosphate ester fostriecin (Figure 1).8
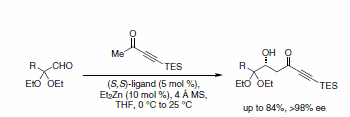
Scheme 7.Alkyne building blocks
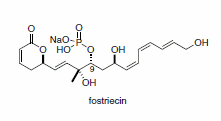
Figure 1.Cytotoxic phosphate ester fostriecin
The nitroaldol (Henry) reaction is an atom economical approach to b-hydroxynitroalkanes that are useful intermediates in the preparation of nitrogen-containing natural products. The asymmetric synthesis of both (–)-denopamine and (–)-arbutamine relied on the dinuclear zinc-catalyzed addition of nitromethane to benzaldehyde derivatives (Scheme 8).9
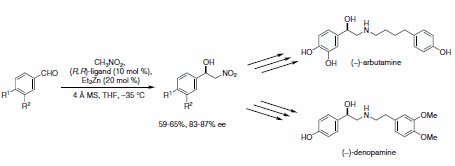
Scheme 8.Benzaldehyde derivatives
Chiral propargylic alcohols are easily accessed in high enantiomeric excess by Zn-catalyzed addition of terminal alkynes to unsaturated aldehydes. This reaction tolerates a variety of alkyne substituents (e.g., Ph, TMS, CO2Et, and CH(OEt)2), as well as both aryl- and α,β-unsaturated aldehydes (Scheme 9).10 In this case, a third equivalent of Et2Zn is neccessary for the formation of the zinc acetylide nucleophile.
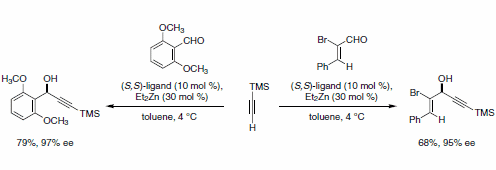
Scheme 9.Unsaturated aldehydes
Finally, the Trost catalyst system efficiently desymmetrizes meso-2-arylpropane-1,3- diols in high-yield with an excellent degree of stereodiscrimination.11 Of the derivatizing agents explored, vinyl benzoate proved most effective in the desymmetrization reaction (Scheme 10).
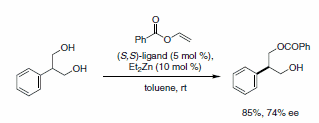
Scheme 10.Desymmetrization reaction
We are pleased to announce an agreement with Professor Barry Trost to distribute this versatile ligand for research applications in Zn-mediated asymmetric C–C bond forming reactions (Scheme 11).
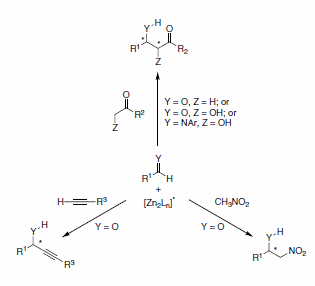
Scheme 11.C–C bond forming reactions
References
To continue reading please sign in or create an account.
Don't Have An Account?