COMU-Safer and More Efficient Peptide Coupling Reagent
Dr. Matthias Junkers
Proteins and peptides are ubiquitous in all living systems. The chemical synthesis of peptidic structures for scientific research or drug discovery relies heavily on efficient coupling reagents. This is especially true for solid phase peptide synthesis—only reagents that yield quantitative results with short reaction times allow the economical synthesis of large peptides. Furthermore, a low tendency for racemization is a key requirement.
A plethora of methods for the formation of the amide bond have been reported. The most successful approaches known today involve active ester formation with uronium/guanidinium salts. The most popular members of this family are peptide synthesis reagents based on a zobenzotriazole and benzotriazole derivatives, such as HOBt or HOAt, both of which are also commonly used as additives in carbodiimide-mediated peptide coupling (Scheme 1).
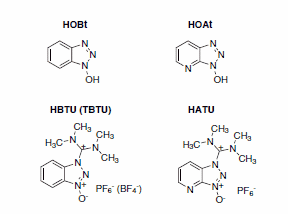
Scheme 1.A zobenzotriazole and benzotriazole based coupling reagents and additives.
HBTU, TBTU, and HATU are the most common peptide coupling reagents based on HOBt and HOAt. How can these successful reagents be improved further? Recent findings in the groups of Fernando Albericio in Spain and Ayman El-Faham in Egypt, showed that the incorporation of a hydrogen bond acceptor in the iminium part of the coupling reagent resulted in a significant performance improvement. Replacing one dimethylamino moiety with a more polar morpholino group proved to enhance stability, solubility, and reactivity of the reagent.1
Some safety concerns also lead to a strong demand for improvements. HOBt derivatives have generally been regarded as potential explosives. Recent transportation reclassifications have made economical shipping and storage increasingly difficult. Currently, HOBt can only be offered commercially in the form of a hydrate. In search for efficacious replacements for benzotriazoles, the Albericio and El-Faham groups demonstrated that ethyl (hydroxyimino)cyanoacetate (Oxyma) is a potent alternative to HOBt or HOAt (Scheme 2).2
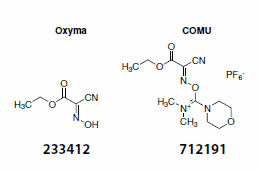
Scheme 2.Oxyma and COMU
In direct comparison to HOBt and HOAt, ethyl (hydroxyimino) cyanoacetate (Oxyma) showed a remarkable capacity to inhibit racemization as an additive in carbodiimide-mediated amide bond formation. Impressively, its coupling efficiency in solid and liquid phase peptide coupling is superior to HOBt, and at least comparable to HOAt.2 DSC and ARC studies of Oxyma show only low thermal risks.
Combining the above findings—the advantageous influence of a morpholino group in the peptide coupling reagent, and the potency of ethyl (hydroxyimino)cyanoacetate (Oxyma) to substitute the benzotriazole moiety as a leaving group—the groups of Albericio and El-Faham dveloped COMU (712191) as a safer and more efficient coupling reagent.3 Is this rising star able to beat its predecessors?
HBTU, HATU, and similar peptide coupling reagents based on benzotriazoles predominantly exist in the less reactive guanidinium or N-form, which is less reactive than the uronium or O-form.4 (Scheme 3)
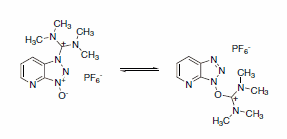
Scheme 3.Guanidinium and uronium form of HATU (also called N- and O-form)
Notably, COMU solely exists as the more reactive uronium structure. Comparative studies proved that COMU exhibits a similar capacity as peptide coupling reagent as the current gold standard, HATU.3 As an outstanding example, the highly demanding synthesis of the Aib-analog of the Leu-enkephalin pentapeptide H-Tyr-Aib-Aib-Phe-Leu-NH2 impressively underlines the superior qualities of COMU. Using COMU, allows the desired product to get synthesized with quantitative yields. Only traces of deletion sequences are found while other coupling reagents including HBTU and HATU, give considerably poorer results. (Table 1)
In a recent investigation Albericio and El-Faham could also show that COMU is fully compatible with microwave-assisted peptide synthesis. Using the same model synthesis of the Aib-analog of the Leu-enkephalin pentapeptide as above COMU clearly outperformed other coupling reagents like HATU or HBTU in direct comparison.5
COMU features optimal properties as a peptide coupling reagent. In addition to its high and fast coupling efficiency, it shows very low or non-existent tendencies for racemization. Epimerization during fragment coupling appears to be lessened with COMU than with HOBt or HATU. COMU is very soluble with remarkable stability in most commonly employed peptide coupling solvents, such as DMF or NMP, which makes it ideally suited for solid phase peptide synthesis. It is equally attractive for solution phase synthesis since by-products formed by COMU are water-soluble and can be separated by simple extraction. A color change during the reaction allows visual or colorimetric reaction monitoring (Figure 1). DSC and ARC data indicate a substantial reduction in the likelihood of a thermal event with COMU.
COMU can be used with nearly identical protocols that apply for common coupling reagents such as HBTU, TBTU, PyBOP, or HATU. Dedicated procedures for the utilization of COMU in solution and solid phase peptide synthesis have been published.6 In circumstances where racemization is a major concern, COMU allows modifications of typical protocols: COMU's morpholino group acts as an internal base during the coupling reaction. Consequently, the addition of external base may be reduced from two to one equivalent. Additionally, the commonly applied DIEA (diisopropylethylamine) can be replaced by the less basic TMP (2,2,6,6-tetramethylpiperidine).
Advantages of COMU
- Equal or even superior performance to HATU
- Non-explosive (does not contain benzotriazole moiety)
- Equally suitable for both solution and solid phase peptide synthesis
- Suitable for microwave-assisted solid phase peptide synthesis
- Utmost retention of configuration–low to non-existent racemization observed
- High solubility and stability in all typical solvents
- Visual or colorimetric reaction monitoring possible
- Easy removal of water-soluble by-products
References
To continue reading please sign in or create an account.
Don't Have An Account?