Azide Building Blocks for Chemical Ligation
Dr. Matthias Junkers
Chemfiles, Volume 10 Article 2
Chemoselective ligation strategies are a key success factor for chemical biology research. Ligation techniques open pathways to fully synthetic large peptides and even proteins. They have proven to be valuable for peptide cyclization. Beside peptide synthesis, carbohydrate synthesis and glycobiology profit equally from further expansion of the chemical ligation toolbox. Generally, chemical ligation allows the modification of biomolecules and biopolymers giving access to hybrid molecules. Molecular probes can be attached selectively to biological targets, some even in living cells, visualizing the complex network of biological interactions. The immobilization of biomolecules on solid supports or nanoparticles facilitates the direct study of their function and structure. The use of protein biochips in high-throughput screens provides a fast gain of insight for advanced biomedical applications.1
Among the plethora of methods for chemical ligation some recent developments stand out.2 A key requirement for the application in biological systems are working conditions at ambient temperature in aqueous media. Furthermore, techniques should be preferred that utilize functional groups usually not present in biological systems. The term “bioorthogonal” was suggested by Prescher and Bertozzi describing this approach.3
Azides satisfy all of these requirements and consequently enjoy growing popularity in recent times. Importantly, the azide group is small and therefore only minimally perturbs natural functions. A whole family of ligation techniques is based on the Staudinger reaction in which azides are reacted with triarylphosphines to form iminophosphoranes with concomitant elimination of nitrogen. (Scheme 1)
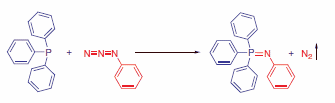
Scheme 1.Staudinger Reaction
To prevent hydrolysis to the primary amine the iminophosphorane needs to be intercepted with an electrophile. Diphenylphosphinoterephthalates offer an intramolecular trap leading to a stable ligation product.4 The activated terephthalate (679011) can be used to introduce any molecular probe into azide group bearing biomolecules (Scheme 2).
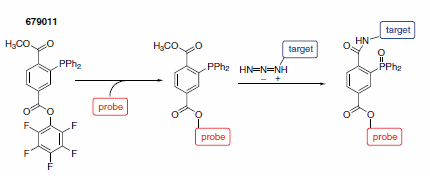
Scheme 2.Nontraceless Staudinger ligation
Traceless versions of this Staudinger ligation developed by Raines and co-workers produce native amide bonds without the inclusion of unnatural phosphine moieties in the final molecule. Thus, the total chemical synthesis of proteins and glycopeptides is enabled overcoming the limitations of native chemical ligation (NCL) of a cysteine residue at the ligation juncture. (Scheme 3)
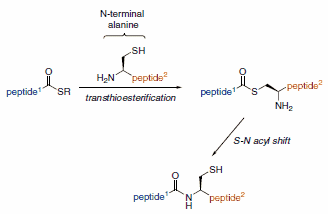
Scheme 3.Native Chemical Ligation
The active Raines reagent needs to be freshly prepared from its shelf stable precursor (670359). The active reagent readily reacts with a thioester activated fragment and links it to an azide fragment without any remaining trace of the reagent in the final molecule. (Scheme 4).
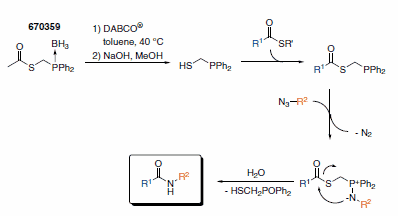
Scheme 4.Traceless Staudinger Ligation
An alternate method to utilize the azide moiety for chemical ligation is the Huisgen 1,3-dipolar cycloaddition reaction with alkynes forming stable aromatic triazoles (Scheme 5).
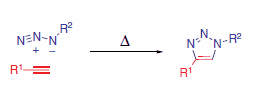
Scheme 5.Huisgen 1,3-dipolar cycloaddition
The copper(I)-catalyzed reaction is mild and very efficient, requiring no protecting groups and no purification in many cases. The resulting triazole exhibits structural similarities to the ubiquitous amide moiety found in natural systems and is completely stable to hydrolysis, oxidation, or reduction. A myriad of applications of the azide alkyne cycloaddition reaction have been published including the modification of metal organic frameworks (MOFs),5 the functionalization of carbon nanotubes,6 protein acetylation monitoring,7 activity based proteome profiling,8 DNA synthesis,9 patterning three-dimensional cell microenvironments,10 or antibody-like protein-capture agents.11 A recent review summarizes applications for biomedical and pharmaceutical polymers.12 Especially for applications within living systems metal-free cycloaddition reactions are desired. Another review highlights current proceedings in this area of research.13
Both the Staudinger reaction family and the azide alkyne cycloaddition reactions require azide building blocks. We are proud to offer the most comprehensive selection of azide modified building blocks for chemical biology with side-chain modified amino acid azides, N-terminal azides, azido monosaccharides and azido polyethylene glycols.
References
To continue reading please sign in or create an account.
Don't Have An Account?