Selection Process Development in a GS Knock-Out CHO Host Cell Line: The Effects of MSX Addition on Clones Generated in an MSX-Free Process
Kate Achtien, Trissa Borgschulte, Nan Lin, Henry George, Kevin J. Kayser
Cell Sciences and Development, SAFC, Sigma-Aldrich, 2909 Laclede Avenue, Saint Louis, MO 63103, USA
Introduction
The Glutamine Synthetase (GS) expression system does not typically require multiple rounds of amplification to isolate high-producing clones (Brown, 1992). However, most Chinese Hamster Ovary (CHO) cell production lines contain endogenous GS, and Methionine Sulfoximine (MSX) is required to inhibit excess GS activity resulting from expression of the endogenous gene and achieve adequate selection. MSX is typically applied during stable pool generation, and is continued at the same concentration throughout the cloning process. A previous study indicates that increasing the concentration of MSX can result in gene amplification and increased productivity (June 2006). However, many of the amplified clones did not stably maintain their enhanced productivity even when cultured in the presence of high concentrations of MSX. Additionally, non-producing clones were shown to survive the amplification process.
Using Zinc Finger Nucleases (ZFNs), we have created a GS -/- host cell line, CHOZN®GS-/-. Since CHOZN®GS-/- lacks functional endogenous GS genes, MSX is not required for selection. We have shown that an MSX-free process can be employed to isolate high-producing clones using this host. The question remains, however, what effects the removal of endogenous GS has on potential amplification methods. In the present study we examine the effects of the addition of MSX to IgG-producing clones developed in an MSX-free process in terms of bulk titer, surface-bound protein expression and gene copy number.
Materials and Methods
Creation of IgG Producing Clones (MSX-Free, ACF Process)
Model IgG-producing stable pools were created using the CHOZN®GS -/- cell line (Figure 1), following the technical bulletin (SAFC). Two pools were single-cell cloned using limiting dilution, and 106 clones were evaluated. 7% of the clones achieved titers of more than 1g/L in a fed batch experiment. Nine high-producing clones were treated with MSX. Stability studies on these clones were carried out in the absence of MSX.
MSX Treatment
The nine IgG-producing clones were placed in media containing 25μM MSX and were cultured in TPP tubes. Following recovery (viability >97%), the MSX-treated clones were banked. During the MSX treatment, the clones were passaged to a VCD of 0.5e6cells/ml twice weekly.
Fed Batch Assay (Volumetric Productivity)
Using the CHOZN®GS platform media and feed system, a fed batch assay was performed on the non-treated and MSX-treated clones, following the CHOZN®GS-/- technical bulletin (SAFC). Titer was measured using the ForteBio Octet Platform.
Surface-Bound IgG
The MSX-treated and non-treated clones were stained using PE conjugated anti-human IgG, washed and analyzed for surface-bound protein levels via MACSQuant Analyzer (Miltenyi Biotec).
Gene Copy Number
The relative copy number of IgG Heavy Chain (HC) and GS genes of the clones was measured via quantitative PCR (Strategene Mx3000P). Relative gene copy numbers were determined and compared using a standard curve created from serially diluted benchmark vector.
Figure 1. Model IgG-producing stable pools were created using the CHOZN® GS -/- cell line
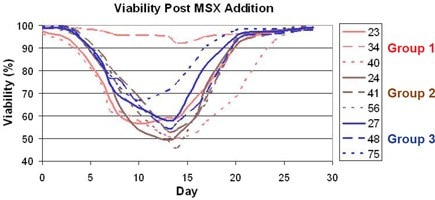
Figure 2.Viability of 9 IgG-producing clones during MSX treatment. Clones are grouped according to changes in productivity post-treatment.
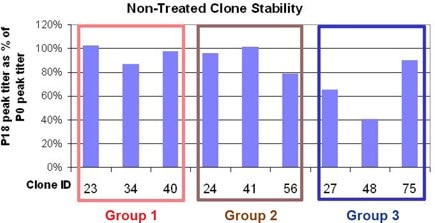
Figure 3.Non-treated clone stability after 18 passages. Passage 18 (P18) titers are shown as a percentage of Passage 0 (P0) titers.

Results and Discussion
Effects of MSX Treatment on Clone Growth (Figure 2)
As shown in Figure 2, each clone responded differently to MSX addition in terms of growth. The minimum viability ranged from 46-92%, and the complete recovery time ranged from 17 to 27 days. No correlation was apparent between growth response to MSX addition and changes in peak titer, gene copy number or surface-bound IgG. For example, two clones that demonstrated the largest increase in volumetric productivity post-MSX treatment had the second lowest (Clone 40) and the highest (Clone 34) minimum viability.
Productivity Changes Following MSX Treatment (Figure 4)
As shown in Figure 4, three clones showed significant increases in titer post-MSXamplification (Group 1). Three clones showed moderate increases (Group 2), and three clones showed reduced volumetric productivity (Group 3).
Relative Gene Copy Number Changes Post-MSX Addition (Figures 5-7)
In Group 1, only one clone showed a significant increase in HC copy number. This clone also showed an increase in GS copy number. In Group 2, no significant changes in HC or GS copy number were observed. In Group 3, two clones showed an increase in GS gene copy number, but a decrease in HC copy number. Overall, the relative gene copy numbers of GS and HC remained constant between the treated and non-treated clones.
Changes in Surface-Bound IgG (Figures 8-10)
In Group 1, all three clones showed a slight increase of surface-bound protein. In one clone, a second, non-producing population emerged post-amplification. In Group 2, one clone showed an increase in surface-bound IgG, but no significant change was observed in the other two clones. In Group 3 all clones showed a second population of non-producing cells that emerged post-amplification, which likely contributed to the decrease in volumetric productivity.
Stability of Non-Treated Clones (Figure 3)
The two clones with the largest decrease in productivity after 18 passages also showed decreased titer after MSX treatment. The surface-bound protein levels for these two clones also show emergent populations of non-producing cells after MSX addition.
Conclusions
- Glutamine (-) selection is sufficient to isolate high-producing clones in CHOZN® GS -/-, but in some cases (3/9 clones in the present study), a 25μM MSX treatment can significantly increase volumetric productivity.
- In most clones, HC copy numbers did not trend with other indicators of gene amplification, such as surface-bound IgG or volumetric productivity; increased IgG productivity may be independent of HC copy number in these clones.
- Two clones demonstrated significant decreases in productivity but moderate increases in GS copy number. This implies that GS and IgG expression are decoupled in these clones, and MSX treatment is therefore ineffective.
- The two clones in which decoupling of GS and IgG expression was indicated also showed decreased titers after 18 passages in the absence of MSX.
- Our results indicate that increases in productivity observed post-MSX treatment are not a direct result of gene amplification. The exact mechanism is not completely understood, however it is apparent that this effect is clone dependent.
Acknowledgements
Ryan Karcher, Scott Bahr, Angie Davis
References
To continue reading please sign in or create an account.
Don't Have An Account?