Tutorial in Microelectronics and Nanoelectronics
Introduction
As the electronic industry continues to move towards smaller and smaller devices, researchers must continually strive for technologies to deposit electronic materials with precision at ever-smaller scales. This trend has lead to a variety of techniques used in the microelectronics industry to layer materials with precision thicknesses, sometimes down to even the atomic level.
Barrier Materials and Precursors
During the manufacturing silicon semiconductor devices, layers of barrier material are deposited to separate interconnecting metal from silicon and prevent their diffusion.
Transition metal nitrides (Product Nos. 333204; TaN, 333247, 595063; TiN, and 402664; NbN) or metal nitride-silicates are widely recognized, used, and examined as barrier materials in ultra-large scale integration of microelectronic devices between copper or aluminum and silicon.1
Barrier materials need to fulfill very strict demands. They must:
- prevent efficient diffusion of metals and silicon through the barrier layer
- not react with the interconnecting metal or silicon
- be stable during the manufacturing and operation of the device
- have low resistivity
- have a good adhesion to the underlying material
- provide good adhesion to deposited metal layer
Titanium nitride TiN has been the most studied barrier material and is used in production at current integration scale. Titanium nitride has several limitations and will probably not meet future demanding requirements for device integrity, (i.e. device failure due to copper diffusion). Intensive research is in progress to identify future barrier materials as well as deposition technique. Tantalum nitride has recently received extensive interest2-4 as a barrier material as well as several other materials:
Ta, TaNx, WNx, TiSixNy, WSixNy, WBxNy5
According to published data, tantalum nitride provides superior physical properties with respect to titanium nitride. It has a high melting point, is very hard, highly conductive, and thermodynamically very stable with respect to Cu because it does not form copper-tantalum or copper-nitride compounds.6,7 As compared to TiN, the grain boundaries of TaN are often disordered while CVD-deposited TiN films typically exhibit a co-lumnar grain structure.8 Due to this disordered grain boundary structure, TaN may prevent copper diffusion more efficiently than TiN.
Several different techniques are used for deposition of barrier layers. Reactive sputtering is the oldest technique, and it has been the main process for depositing tantalum nitride films. Sputtered films are usually quite free from impurities and have low resistivity, but the step coverage is poor.
Chemical vapor deposition (CVD) methods are a main techniques currently used for deposition of metal nitride layers for different applications. The films made by CVD exhibit much better conformity than the films deposited by physical vapor deposition methods.
Conventional CVD processes, which rely on metal halides, required high temperature (for example, deposition of tantalum nitride, TaN, from TaCl5 required 900 °C).9 This problem has been solved by using MOCVD precursors10,11 and low temperature plasma enhanced chemical vapor deposition (PECVD).
The other variant of deposition process, atomic layer deposition (ALD) and its several enhancements, allows very good control of layer thickness and uniformity, provides a good step coverage, and relatively low deposition temperature. It is worthy to notice that thin-film transition metal nitrides have a potential in other applications besides diffusion barriers. In microelectronics, TaN, for example, has been used as a thin-film resistor and as passivation layer against copper oxidation.4 It has been considered as an electrode material for those dynamic random access memory (DRAM) storage capacitors where tantalum(V) oxide (Ta2O5, Product Nos. 204536, 303518, 383021, 484477, and 484482) is used as a dielectric material.12 Tantalum nitride has also been investigated as a nonmagnetic interlayer in NiFeCo/TaN/NiFeCo nonvolatile magnetic random access memories.13
In addition to microelectronics, TaN films has also been examined for high-temperature ceramic pressure sensors because of their good stability and piezoresistive properties.14 Other potential uses of TaN include hard, protective coatings. Chemical precursors used in barrier deposition processes represent several classes of materials such as metals, metal halides, metal carbonyls, and metal amides.8,12
Our chemists, working in collaboration with leading industrial and academic researchers, have prepared an extensive selection of precursor compounds. These precursor compositions have been purified, in many cases, to 99.9999% on metals basis, and characterized by a variety of analytical methods including ICP, multinuclear-NMR, GC, and ICP-MS. They are already available in research quantities and can be packaged in electro-polished stainless steel containers. When large-scale requirements evolve, we have the manufacturing capacity and scale-up experience to manufacture the products in commercial scale. In addition to established products, we also support the semiconductor industry by offering custom synthesis to address application-specific needs of customers.
High κ Dielectric Materials
Since its inception almost a half century ago, the semiconductor industry has been based on the properties of silicon. Particularly important has been the electrically stable nature of the Si/SiO2/metal materials stack, which defines the capacitor, a basic structure in microelectronics (Figure 1). As integrated circuit design rules shrink, elements of the circuit must be miniaturized. This relentless miniaturization has led to enhanced chip performance in terms of processing speeds and to expanded memory capacities at diminishing unit costs. Previously, limits on miniaturization were largely technological in nature, whereas now the fundamental properties of silicon dioxide itself are restricting further device size reduction.
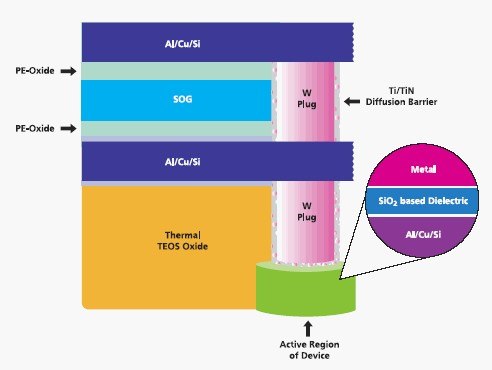
Figure 1.Cross-sectional view of a typical integrated circuit showing details of the Si/SiO2/Metal materials stack forming the gate capacitor structure in the inset.
The limitation of silica (silicon dioxide, SiO2) is in its ability to behave as an electrical insulator, that is, a dielectric barrier. The dielectric strength of a material is expressed in terms of its dielectric constant, κ (for SiO2, κ ≈ 4 and for SiOxNy, κ = 5-6). At the design dimensions contemplated for the future generations of semiconductor devices, the thickness of the dielectric layer of the capacitor will be such that silica derived materials will not have sufficient strength to behave as an effective insulator. Electrical current will leak across the dielectric and the capacitor will discharge. In an effort to remove this obstacle to miniaturization, researchers in semiconductor device architecture and manufacturing have considered alternative materials featuring higher κ values for such critical dielectric films in both the gate electrodes and in DRAM (dynamic random access memory) storage capacitors. Investigators have focused on early transition metal oxides and their silicates (for example, titanium, zirconium and hafnium oxides, TiO2, κ = 60, ZrO2, κ = 23; HfO2, κ = 20 or tantalum oxide, Ta2O5, κ = 25) as well as multi-metallic oxides (for example barium strontium titanate, BST, κ = 300), especially for DRAM applications. Although conceptually simple, this material substitution for SiO2-based materials is complex from a practical and a commercial perspective. Issues associated with material patterning and interfacial stability with respect to other materials in contact with the dielectric (so called "integration questions") in addition to cost of ownership questions for whatever film manufacturing technique demonstrates value remain largely unanswered at this time.
Key material properties of any new high κ materials include high permittivity or dielectric constant, barrier properties to prevent tunneling, stability in direct contact with silicon, good interface quality and good film morphology. The dielectric must also be able to demonstrate its compatibility with the gate material, process compatibility, and high reliability. The more aggressively companies scale transistor dimensions in high-performance devices, the more quickly a high κ material will be needed to replace oxide gate dielectrics.
The absolute limit for oxide/nitride films appears to be 7 Å, but 12 Å may represent the practical limit, since effects of gate leakage or reliability will likely prevent further improvements in device performance.15 The replacement of SiO2 with a high permittivity (high- κ) gate dielectric (for example, HfSixOy, ZrSixOy, Si3N4) presents a formidable challenge to the semiconductor industry. To effectively replace silica, a high κ gate dielectric must exhibit low leakage current, must have a capacitance and performance at least as equivalent to SiO2, must be able to be integrated into a full process flow, and must have sufficient reliability at operating conditions.
Deposition of TiO2, ZrO2, and HfO2 has been study by Campbell and coworkers. Depositions were carried out by CVD process using the respective nitrate precursor M(NO3)4, where M = Ti, Zr, or Hf. Physical and electrical properties of deposited layer and the interface with substrate were investigated. Zirconium and hafnium oxides emerged as a possible candidate for gate material.16
According to the ITRS (International Technology Roadmap for Semiconductor) data the best candidate for gate material should possess κ in the 10 – 25 range. Material for memory capacitors should have much higher κ value. Table 1 shows the relevant properties of some high k dielectric candidates.
Relevant Properties of High-k Dielectric Candidates |
|---|
* Yoon, D.-S.; Roh, J.S. Critical Rev. Solid State Mat. Sci. 2002, 27, 143
Deposition Techniques
Several techniques could be use to deposit layers of high κ material, metal oxides, or silicates. Physical techniques such as physical vapor deposition (PVD) or molecular beam epitaxy (MBE) exhibit problems with wafer throughput and conformatlity. Sputtering techniques can lead to impurities, uniformities, composition control, or damage to the substrate. Chemical solution deposition is not useful for thin films (though it works very well for thick films). Chemical vapor deposition (CVD) works very well for thin layer deposition, particularly atomic layer deposition (ALD).
Chemical Vapor Deposition (CVD)
Several different techniques are used for deposition of barrier layers. Reactive sputtering is the oldest technique, and it has been the main process for depositing tantalum nitride films. Sputtered films are usually quite free from impurities and have low resistivity, but the step coverage is poor.
Chemical Vapor Deposition (CVD) method is the main technique currently used for deposition of metal nitride layers for different applications. The films made by CVD exhibit much better conformal coverage than the films deposited by physical vapor deposition methods.
Good deposition precursors should not only be volatile but should also have predictable decomposition routes, with gaseous or highly volatile side products from decomposition. Metal organic precursors can be designed to meet all these needs, but care must be taken in their synthesis. Our scientists ensure the high-purity standards necessary for MOCVD precursors by using only the highest purity starting materials: metals, metal halides, or organometallic reagents as well as high-purity solvents and organic ligands.
Atomic Layer Deposition (ALD)
Atomic Layer Deposition (ALD) is a surface controlled layer-by-layer process for the deposition of thin films with atomic layer accuracy. Each atomic layer formed in the sequential process is a result of saturated surface controlled chemical reactions.
Chemisorption, that is the chemical reaction between the volatile precursor and the surface, is assured by carefully selecting the reaction temperature so that the precursors are not allowed to condense or decompose on the surface. The precursor doses are kept high enough to achieve surface saturation. Commonly, in the growth of binary compounds such as metal oxides, a reaction cycle consists of two reaction steps. In one step the metal compound precursor is allowed to react with the surface, and in the other step it reacts with the oxygen precursor. Between the steps a purge is applied to remove the excess of precursor and the reaction by-products.
The reaction of trimethylaluminum (TMA, Product Nos. 257222, 462578, and 597775) with water represents a classic example of the ALD-process (Figure 2).

Figure 2.The reaction of trimethylaluminum with water.
The first step of a TMA reaction with an oxidized silicon surface is presented in Figure 3. Surface hydroxyl group and oxygen reacting with Al-C bond with formation of a Si-O-Al linkage.
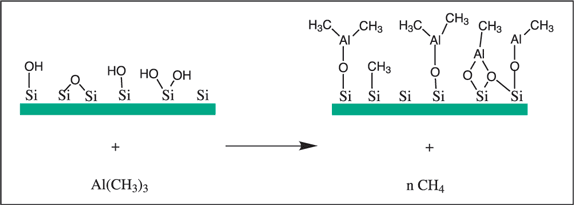
Figure 3.The reaction of trimethylaluminum with an oxidized silicon surface.
In the next reaction cycle water reacts with aluminum methyl groups, regenerating hydroxyl group on the surface evolving of methane (Figure 4).
Figure 4.The reaction of surface alkylaluminum groups with water.
The self-controlled growth mode of ALCVD contributes several advantages. The thickness of the films can be controlled in a straightforward manner by controlling the number of reaction cycles, therefore enabling the controlled growth of ultra thin layers. The precursors are saturatively chemisorbed, thus bringing stoichiometric films with large area uniformity and conformality even on complex surfaces with deformities. Layer-by-layer growth allows one to change the material abruptly after each step. This gives the possibility to deposit multicomponent films (so called nanolaminates or mixed oxides for example, which can than be used for tailoring the electrical properties).17
It is now clear that whatever high κ material is used, a film deposition process, such as chemical vapor deposition (CVD) or its relative, atomic layer deposition (ALD), will likely be employed. Both CVD and ALD require volatile, highly purified, molecular precursors to the desired solid-state compositions of matter. In CVD or ALD a molecular precursor in the vapor phase is transported to the surface of a wafer where chemical transformation occurs leaving the desired solid-state composition as a thin film and releasing volatile coproducts that exit the reactor.
In order to enable the development of this critical new technology, the semiconductor industry needs a source for a large selection of suitable molecular precursors (such as early transition metals and lanthanide alkyls, amides, alkoxides, β-diketonates, and nitrates). Our chemists, working in collaboration with leading industrial and academic researchers, have prepared an extensive selection of such compounds. We recognize that speed to market is a critical success factor in commercializing new technology in the semiconductor industry. Accordingly, we support the industry by providing flexible production capacity, from kilos to metric tons, for such compounds, many of which require anærobic or low temperature (down to –110 ºC) manufacturing methods. Strict adherence to reactor cleaning protocols enables large-scale production at low trace element contamination levels. In addition, we have a separate manufacturing facility dedicated to the production of ultra-high purity inorganic salts, common starting materials in precursor synthesis, thereby establishing control over product purity throughout the process.
References
To continue reading please sign in or create an account.
Don't Have An Account?