Monocyte Activation Test (MAT) in Vitro Test Kits for Pyrogen and Endotoxin Testing
Testing for pyrogens is a critical step in ensuring the safety of parenteral pharmaceutical products and medical devices. The Monocyte Activation Test (MAT) can detect both endotoxin and non-endotoxin pyrogens in one test. Supported by many regulatory bodies, the robust MAT assay produces sensitive results based on the human immune reaction.
Comparison of rabbit, LAL and pyrogen tests

Figure 1.Cell line-based pyrogen test which mimics the human immune reaction.
The PyroMAT® System is based on the Mono-Mac-6 (MM6) cell line and uses Interleukin-6 (IL-6) as a read out. Once these human monocytes come into contact with pyrogens from a contaminated sample, they produce cytokines such as Interileukin-6 (IL-6), which can be detected with an enzyme-linked immunosorbent assay (ELISA).
Figure 1.Pyromat system
All the advantages of the monocyte activation test combined with the benefits of using a cell line.
- Detection of a broad range of pyrogens: patient safety is ensured if the full range of pyrogens are tested. Like the Rabbit Pyrogen Test (RPT), MAT is effective for both endotoxin and non-endotoxin testing.
- Extension of the range of products that can be tested: the most frequently applied methods, RPT, Bacterial Endotoxin Test (BET) or LAL, are limited in the product types they are able to test. The MAT offers more flexibility regarding its applications.
- In vitro assay that mimics the human immune reaction: for a robust predictive model that reduces animal consumption.
- Compliance with international regulations and guidelines: in line with ethical trends of industry and regulatory authorities to decrease the use of animal-based testing.
- Standardized reactivity and high sensitivity (LOD 0.05 EU/mL). Convenience of a ready-to-use cell line reduces laborious lab work and avoids the need for a cell culture laboratory.
- Qualified cells: in addition to being cited in the international validation of MAT, Mono-Mac-6 cells are qualified for the detection of all types of pyrogens by testing the expression of all surface Toll-Like Receptors (TLRs).
How to Use the PyroMAT® System to Test for Endotoxin and Non-endotoxin Pyrogens
The PyroDetect system: a non-animal alternative to the rabbit test and the LAL test
The PyroDetect system was the first Monocyte Activation Test (MAT) kit available on the market. Our PyroDetect system uses cryoblood (frozen human whole blood) pooled from donors as a source of monocytes and Interleukin-1β as the ELISA read-out.
Figure 2. PyroDetect system
How the PyroDetect system works
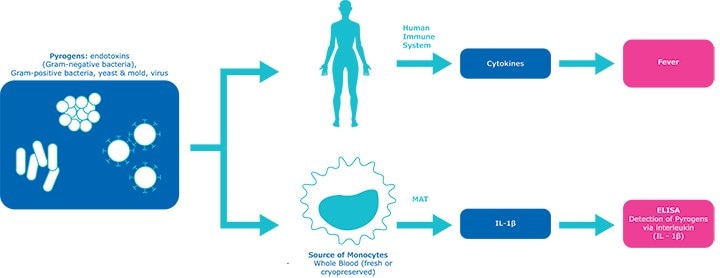
Figure 3.How the PyroDetect system works
PyroDetect Key Benefits
- Detection of a wide spectrum of pyrogens: like the Rabbit Pyrogen Test (RPT), the MAT is able to detect both endotoxins and NEPs.
- Extended range of products that can be tested: the most frequently applied methods, RPT, Bacterial Endotoxin Test (BET) and LAL, are limited in the product types they can be used with. The MAT offers greater flexibility regarding its applications.
- An in vitro assay that mimics the human immune reaction: for a robust predictive model that reduces animal consumption.
- Compliance with international regulations and guidelines: in line with ethical trends of industry and regulatory authorities to decrease the use of animal-based testing.
- Cryopreserved blood pooled from 8 donors: to be as close as possible to the human immune reaction to pyrogens.
To continue reading please sign in or create an account.
Don't Have An Account?