Raw Material Risk Mitigation Strategies
Cell Sciences and Development, Lenexa, KS, USA
Introduction
Media variability is a major concern for the biopharmaceutical industry. A wideranging program to evaluate and understand the variability is critical to produce consistent quality cell culture products. Raw Material Characterization (RMC) was launched to fulfill the unmet need in the industry.
Figure 1.Complexity of inputs in a medium product.
Description
The Need and the Approach
To understand and address the changing nature of supply chain, a risk-based process was established. This was achieved by integrating the RMC program into the global supplier quality initiative. The integration allowed a risk-based approach to evaluate raw material risks.
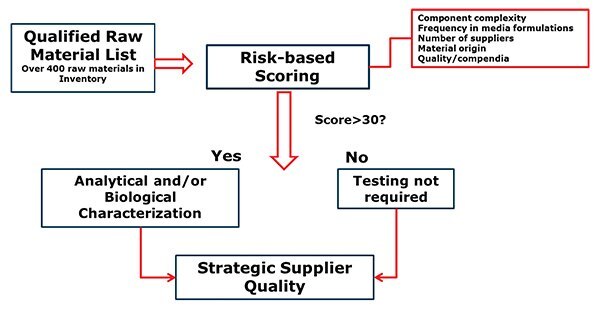
Figure 2.Risk based approach to assess supplier change notifications.
Trace Elements and Beyond
Trace element impurities in culture media have been implicated in causing variability in biopharmaceutical processes. The types of trace elements, the levels at which they impact culture performance and the source of these impurities has not been well characterized. RMC has a three pronged approach to study trace element variability.
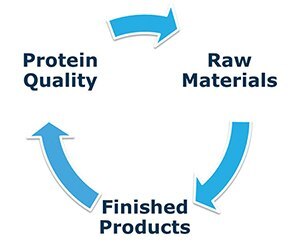
Figure 3.Approach to study trace element impurities; single components, culture medium and the impact on protein quality.
Results and Discussion
Risk-Based Scoring
The approach shown in Figure 2 has allowed the team to assess hundreds of raw materials and perform additional testing if needed. The testing may require developing novel analytical and bio assays (Poloxamer 188). We have rejected five raw materials (Figure 4) before being used in products. A similar approach is also used to assess viral risk.

Figure 4.The chart shows total number of raw materials considered for risk evaluation and mitigation.
Trace Element Variability
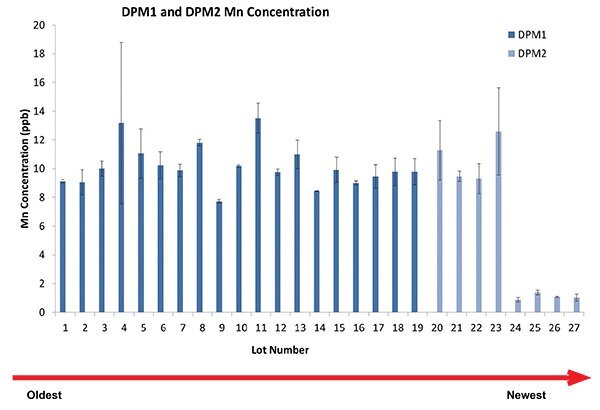
Figure 5.Impact of a single raw material in final Mn level.
Figure 5 shows the impact of a cell culture component (vitamin B6) in the final concentration of Mn. The total concentration of this vitamin was 0.2% by weight. In-house models showed that this change in Mn concentration didn’t have any impact on protein quality. Though, Mn at higher concentrations showed an increase in galactosylation (data not shown).
Conclusions
Raw material variability is inevitable. Both suppliers and consumers of cell culture products need a systematic approach to asses and mitigate the risks. Risk factors can include supply chain changes, trace metal impurities and viral risks.
Summary
The raw material characterization (RMC) program is an original initiative designed to address the changing environment in the biopharmaceutical industry. Initially an internal effort to study variability across raw materials used for cell culture media, the program has now evolved into an integral part of critical raw material management for the organization. The focal point of the program is to understand, evaluate and mitigate risks brought in by cell culture components.
To continue reading please sign in or create an account.
Don't Have An Account?