All Photos(1)
About This Item
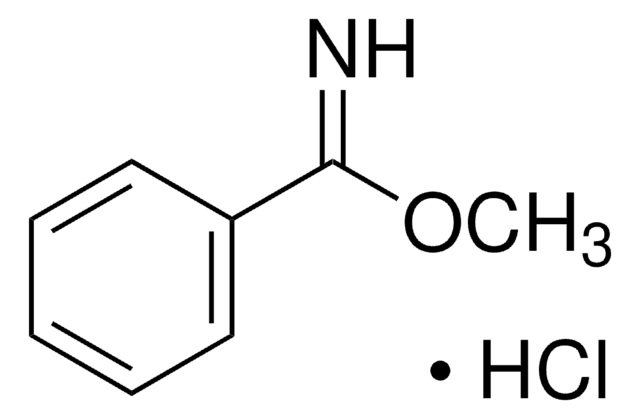
Linear Formula:
C6H5C(=NH)OCH2CH3 · HCl
CAS Number:
Molecular Weight:
185.65
Beilstein/REAXYS Number:
3913195
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
≥97.0% (AT)
mp
~125 °C (dec.)
SMILES string
Cl[H].CCOC(=N)c1ccccc1
InChI
1S/C9H11NO.ClH/c1-2-11-9(10)8-6-4-3-5-7-8;/h3-7,10H,2H2,1H3;1H
InChI key
MODZVIMSNXSQIH-UHFFFAOYSA-N
Related Categories
General description
Ethyl benzimidate hydrochloride reacts with (R)-ethyl cysteine hydrochloride in ethanol to yield (4R)-ethyl 2-phenyl-4,5-dihydrothiazole-4-carboxylate. It reacts with D-Penicillamine methyl ester hydrochloride and triethylamine to yield methyl-5,5- dimethyl-2-phenyl-2-thiazoline-4-carboxylate.
Application
Intermediate for synthesis
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The Synthesis of Substituted Penicillins and Simpler Structural Analogs. III. Phthalimido ?-Lactam-Thiazolidines Derived from Penicillamine.
Sheehan JC, et al.
Journal of the American Chemical Society, 73(9), 4373-4375 (1951)
Satendra Singh et al.
The Journal of organic chemistry, 69(13), 4551-4554 (2004-06-19)
(1R)-(+)-2,10- and (1S)-(-)-2,10-camphorsultam were acylated with ethyl 2-phenylthiazoline 4-carboxylate to afford (+)- and (-)-2-phenylthiazolinylcamphorsultam, which were stereoselectively alkylated with MeI in the presence of n-BuLi. Alkylation of these phenylthiazolinylcamphorsultams occurred from the beta-face rather than alpha-face, resulting in the formation
Aerobic dissipation of the novel cyanoacrylate fungicide phenamacril in soil and sludge incubations.
Søren S Donau et al.
Chemosphere, 233, 873-878 (2019-07-26)
The cyanoacrylate ethyl (2Z)-3-amino-2-cyano-3-phenylacrylate (phenamacril), has been introduced as an effective agent against several fungi species belonging to the Fusarium genus. However, in current literature, knowledge about the environmental behavior of this fungicide is limited and there are no data
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service