146293
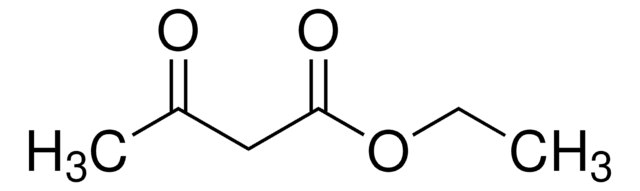
Ethyl (4-fluorobenzoyl)acetate
Synonym(s):
(p-Fluorobenzoyl)acetic acid ethyl ester, 4-Fluoro-ß-oxobenzenepropanoic acid ethyl ester, Ethyl 3-(4-fluorophenyl)-3-oxopropionate, Ethyl 4-fluorobenzoylacetate
About This Item
Recommended Products
refractive index
n20/D 1.5040 (lit.)
Quality Level
bp
117-120 °C (lit.)
density
1.174 g/mL at 25 °C (lit.)
SMILES string
CCOC(=O)CC(=O)c1ccc(F)cc1
InChI
1S/C11H11FO3/c1-2-15-11(14)7-10(13)8-3-5-9(12)6-4-8/h3-6H,2,7H2,1H3
InChI key
SJUXLKYJKQBZLM-UHFFFAOYSA-N
General description
Application
- Condensation reactions with diamines via C-C bond cleavage for synthesis of benzimidazoles and perimidines for possible use as antimalarial treatments
- Base-promoted domino Michael addition / cyclization / elimination reactions for synthesis of hydroxybenzophenones
- Oxidative cross-coupling with indoles via dioxygen activation
- Cyclization of keto esters for synthesis of pyrones
- Lewis base catalyzed hydrosilylation for synthesis of α-acetoxy β-amino acid derivatives
- Conia-ene reactions for synthesis of methylenecyclopentane derivatives
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service