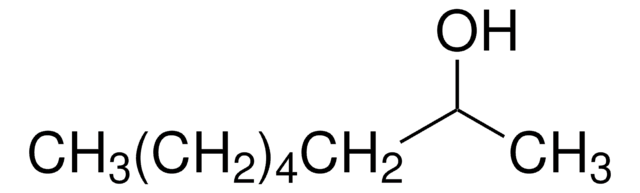About This Item
Recommended Products
Quality Level
assay
99%
form
liquid
optical activity
[α]20/D +9.5°, neat
optical purity
ee: 98% (GLC)
refractive index
n20/D 1.426 (lit.)
bp
175 °C (lit.)
density
0.822 g/mL at 25 °C (lit.)
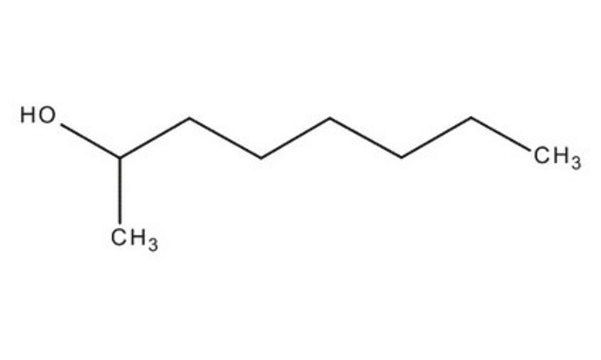
SMILES string
CCCCCC[C@H](C)O
InChI
1S/C8H18O/c1-3-4-5-6-7-8(2)9/h8-9H,3-7H2,1-2H3/t8-/m0/s1
InChI key
SJWFXCIHNDVPSH-QMMMGPOBSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- To prepare the solution of 5-(benzyloxy)-isophthalic acid derivative, which is used as a 2D chiral self-assembly system comprising pyrolytic graphite.
- As a chiral template in the study of enantioselective glycidol esterification.
- As a starting material for the preparation of (+)-(S)-2-octyI tosylate, an intermediate used to prepare (−)-(R)-2-halo and azido octanes.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2
wgk_germany
WGK 3
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service