151637
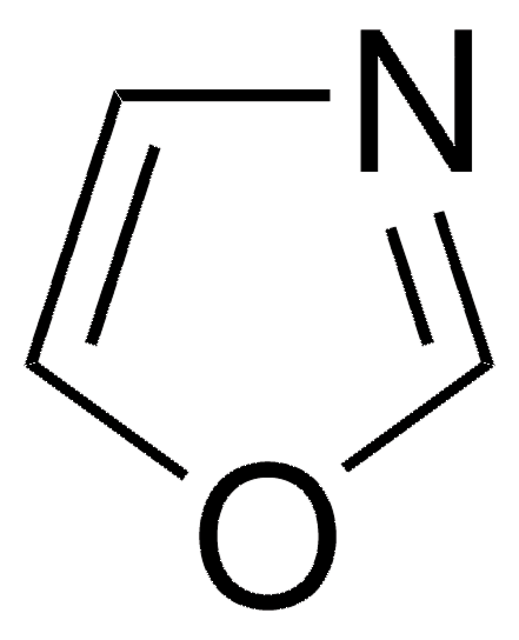
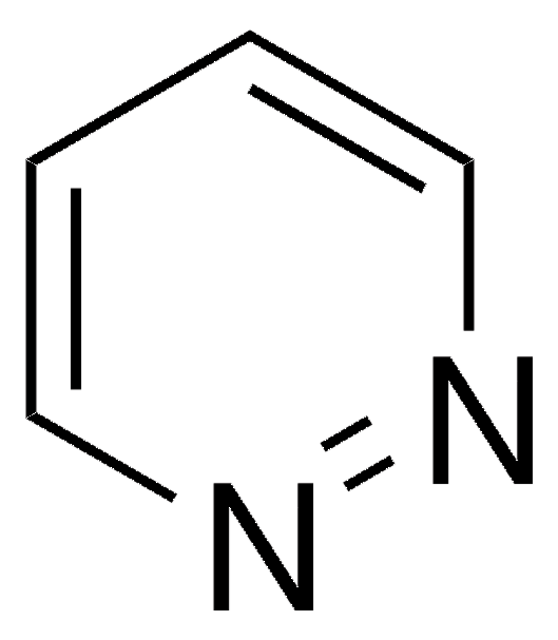
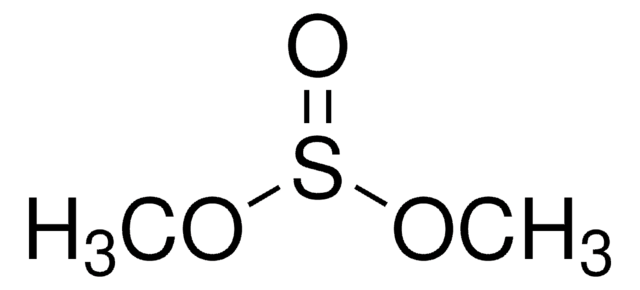
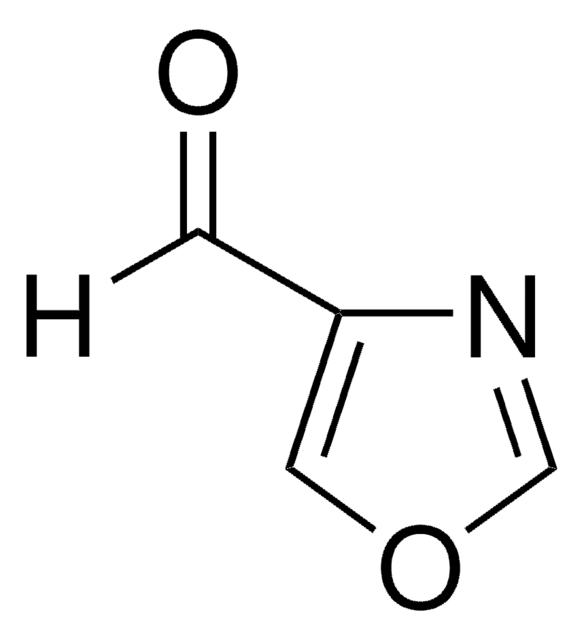
Isoxazole
99%
Synonym(s):
1,2-Oxazole, 1-Oxa-2-azacyclopentadiene, 2-Azafuran
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C3H3NO
CAS Number:
Molecular Weight:
69.06
Beilstein/REAXYS Number:
103773
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
2.4 (vs air)
Quality Level
assay
99%
form
liquid
refractive index
n20/D 1.427 (lit.)
bp
93-95 °C (lit.)
density
1.078 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
c1cnoc1
InChI
1S/C3H3NO/c1-2-4-5-3-1/h1-3H
InChI key
CTAPFRYPJLPFDF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Isoxazole are described as inhibitors of acetylcholinesterase (AChE). Isoxazole ligands bind to and inhibit the Sxc- antiporter.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Margarita Gutiérrez et al.
The Journal of pharmacy and pharmacology, 65(12), 1796-1804 (2013-11-05)
Inhibition of acetylcholinesterase (AChE) is a common treatment for early stages of Alzheimer's disease. In this study, nine isoxazoles derivatives were tested for their in-vitro AChE activity. The molecular docking showed the interaction of the compounds with the active site.
Mingxing Wang et al.
Journal of molecular graphics & modelling, 84, 18-28 (2018-05-25)
Studies on human genetics have implicated the voltage-gated sodium channel Nav1.7 as an appealing target for the treatment of pain. In this study, we put forward a ligand-based pharmacophore for the first time, which was generated by a set of
Marina N Semenova et al.
ACS combinatorial science, 20(12), 700-721 (2018-11-20)
A series of both novel and reported combretastatin analogues, including diarylpyrazoles, -isoxazoles, -1,2,3-triazoles, and -pyrroles, were synthesized via improved protocols to evaluate their antimitotic antitubulin activity using in vivo sea urchin embryo assay and a panel of human cancer cells.
Adam A Wallace et al.
The journal of physical chemistry. A, 125(1), 317-326 (2020-12-29)
Electron capture by the σ* LUMO of isoxazole triggers the dissociation of the O-N bond and the opening of the ring. Photodetachment of the resulting anion accesses a neutral structure, in which the O· and ·N bond fragments interact through
Sahaya Asirvatham et al.
Anti-inflammatory & anti-allergy agents in medicinal chemistry, 14(2), 128-137 (2015-08-13)
A series of newer 3-(4'-methoxyphenyl)-5-substituted phenylisoxazoles derivatives have been synthesized by reacting hydroxylamine hydrochloride with chalcones. The chalcones were formed by reacting different aromatic aldehydes with 4-methoxyacetophenone in presence of aqueos potassium hydroxide (KOH). The purity of all the synthesized
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service