155071
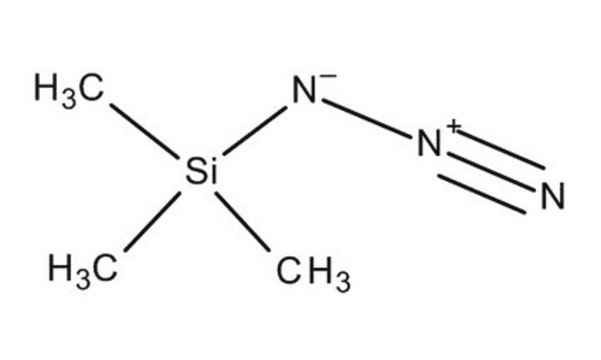
Azidotrimethylsilane
95%
Synonym(s):
Trimethylsilyl azide
About This Item
Recommended Products
Quality Level
assay
95%
form
liquid
refractive index
n20/D 1.414 (lit.)
bp
52-53 °C/175 mmHg (lit.)
density
0.868 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C[Si](C)(C)N=[N+]=[N-]
InChI
1S/C3H9N3Si/c1-7(2,3)6-5-4/h1-3H3
InChI key
SEDZOYHHAIAQIW-UHFFFAOYSA-N
Related Categories
General description
Application
- A nitrogen precursor to prepare GaN nanowire via metal-organic chemical vapor deposition method.
- An electrolyte additive in Li-O2 batteries. The addition of TMSN3 results in the formation of robust solid electrolyte interphase.
- An efficient reagent in the synthesis of tetrazoles, fullerenyl azide, and α-azido oximes.
- A silylating agent in the O-trimethyl silylation of alcohols and phenols.
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
42.8 °F - closed cup
flash_point_c
6 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Click chemistry, and the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) in particular, is a powerful new synthetic tool in polymer chemistry and material science.
The chemistry of organoazides is exceedingly rich, since the azide functionality reacts with electrophiles, nucleophiles, and dipolarophiles, with or without the extrusion of dinitrogen. Common place transformation such as Staudinger reductions or ligations, Cu(I)-catalyzed Huisgen cycloadditions (of the “click” reaction family), Curtius or Schmidt rearrangents, nitrene reactions, or imine formation via aza-Wittig reactions all necessitate organoazide precursors or intermediates
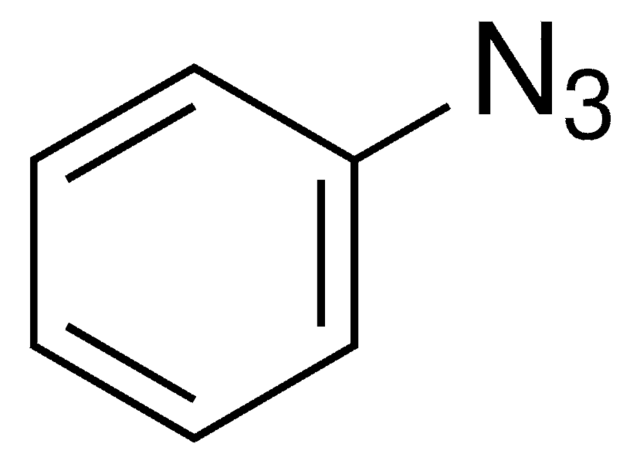
Since the preparation of the first organic azide, phenyl azide, by Peter Griess in 1864 this energy-rich and versatile class of compounds has enjoyed considerable interest.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service