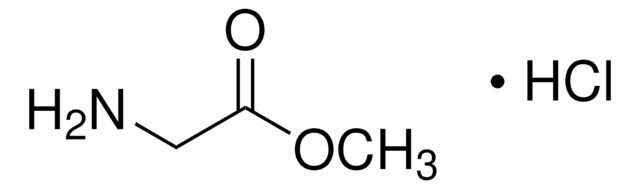
15512
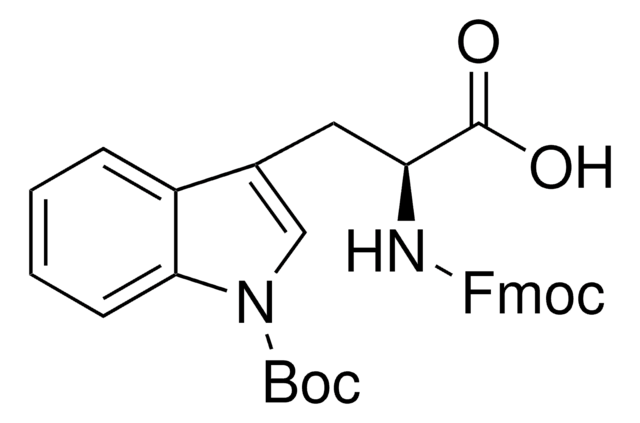
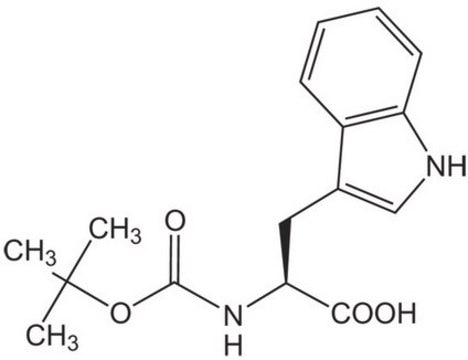
Boc-Trp-OH
≥99.0% (TLC)
Synonym(s):
Nα-(tert-Butoxycarbonyl)-L-tryptophan, Nα-Boc-L-tryptophan
About This Item
Recommended Products
Quality Level
assay
≥99.0% (TLC)
optical activity
[α]20/D −20±1°, c = 1% in DMF
reaction suitability
reaction type: Boc solid-phase peptide synthesis
mp
136 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
CC(C)(C)OC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O
InChI
1S/C16H20N2O4/c1-16(2,3)22-15(21)18-13(14(19)20)8-10-9-17-12-7-5-4-6-11(10)12/h4-7,9,13,17H,8H2,1-3H3,(H,18,21)(H,19,20)/t13-/m0/s1
InChI key
NFVNYBJCJGKVQK-ZDUSSCGKSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service