All Photos(1)
About This Item
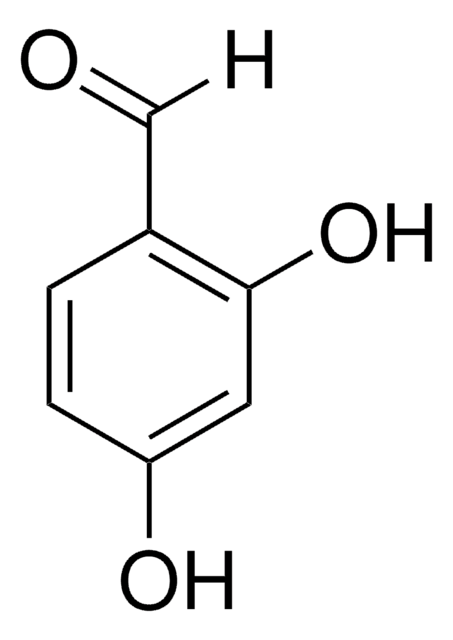
Linear Formula:
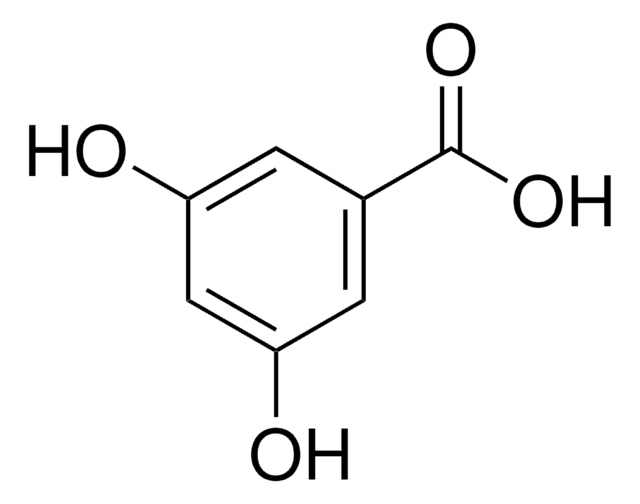
(HO)2C6H3CO2CH3
CAS Number:
Molecular Weight:
168.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
form
solid
mp
167-170 °C (lit.)
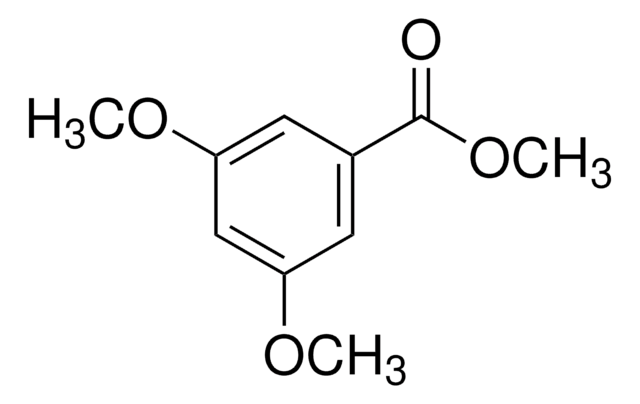
SMILES string
COC(=O)c1cc(O)cc(O)c1
InChI
1S/C8H8O4/c1-12-8(11)5-2-6(9)4-7(10)3-5/h2-4,9-10H,1H3
InChI key
RNVFYQUEEMZKLR-UHFFFAOYSA-N
Related Categories
General description
Ribonucleotide reductase inhibiton and antiumor activity of methyl 3,5-dihydroxybenzoate has been studied.
Application
Methyl 3,5-dihydroxybenzoate was used in the synthesis of cored dendrimers. It was also used in the preparation of bis(5-carbomethoxy-1,3-phenylene)-32-crown-10, a semi-rigid 32-membered ring diester crown ether.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of cored dendrimers.
Wendland MS and Zimmerman SC.
Journal of the American Chemical Society, 121(6), 1389-1390 (1999)
Macrocyclic polymers. 1. Synthesis of a poly (ester crown) based on bis (5-carboxy-1, 3-phenylene)-32-crown-10 and 4, 4'-isopropylidenediphenol (bisphenol A).
Delaviz Y and Gibson HW.
Macromolecules, 25(1), 18-20 (1992)
New ribonucleotide reductase inhibitors with antineoplastic activity.
H L Elford et al.
Cancer research, 39(3), 844-851 (1979-03-01)
Shota Machida et al.
Molecules (Basel, Switzerland), 24(23) (2019-12-01)
Twenty-one natural and unnatural phenolic compounds containing a carbohydrate moiety were synthesized and their structure-activity relationship (SAR) was evaluated for α-glucosidase inhibition and antioxidative activity. Varying the position of the galloyl unit on the 1,5-anhydro-d-glucitol (1,5-AG) core resulted in changes
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service