198242
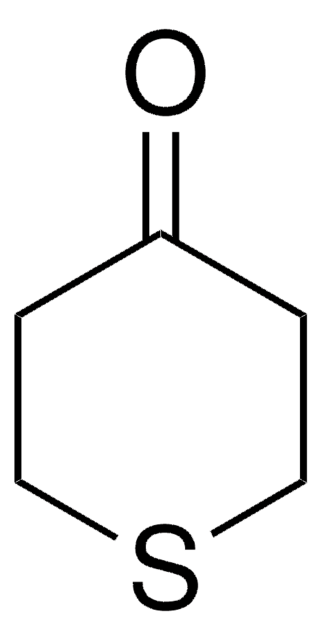
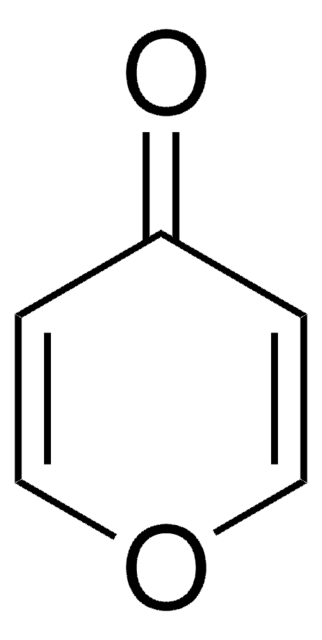
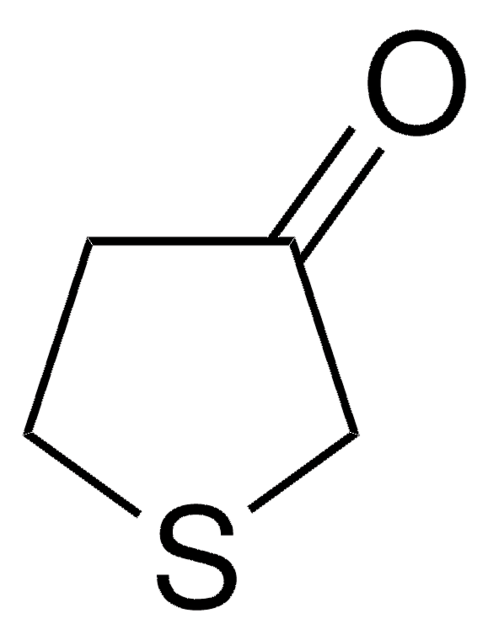
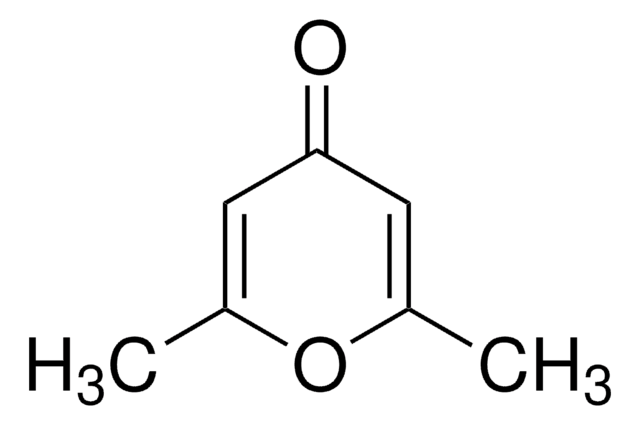
Tetrahydro-4H-pyran-4-one
99%
Synonym(s):
4-Oxotetrahydropyran
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H8O2
CAS Number:
Molecular Weight:
100.12
Beilstein/REAXYS Number:
106463
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
99%
form
liquid
refractive index
n20/D 1.452 (lit.)
bp
166-166.5 °C (lit.)
density
1.084 g/mL at 25 °C (lit.)
SMILES string
O=C1CCOCC1
InChI
1S/C5H8O2/c6-5-1-3-7-4-2-5/h1-4H2
InChI key
JMJRYTGVHCAYCT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
R K Anderson et al.
The Journal of antibiotics, 46(2), 331-342 (1993-02-01)
Syntheses are described for penicillins (4b approximately 4i, 5a and 5b) which possess a 6 beta-(2-heteroaryl-3-substituted)-propenamido side-chain of fixed geometry. In vitro results for these compounds against a range of Gram-positive and Gram-negative bacteria showed in most cases good stability
Kun Huang et al.
The Journal of organic chemistry, 71(21), 8320-8323 (2006-10-10)
As the first example for the synthesis of optically active alpha-hydroxyaldehydes and alpha-hydroxyketones in ionic liquids, we applied RTILs into L-proline catalyzed direct enantioselective alpha-aminoxylation of both aldehydes and ketones successfully. This protocol features a number of advantages, such as
Synthesis, 672-672 (1994)
Journal of Heterocyclic Chemistry, 31, 397-397 (1994)
Bryan Ringstrand et al.
Beilstein journal of organic chemistry, 7, 386-393 (2011-04-23)
The methodology to prepare 3-substituted 1,5-dibromopentanes I and their immediate precursors, which include 3-substituted 1,5-pentanediols VII or 4-substituted tetrahydropyrans VIII, is surveyed. Such dibromides I are important intermediates in the preparation of liquid crystalline derivatives containing 6-membered heterocyclic rings. Four
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service