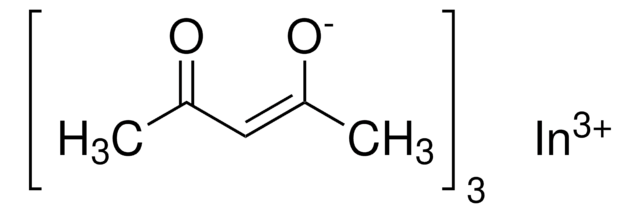
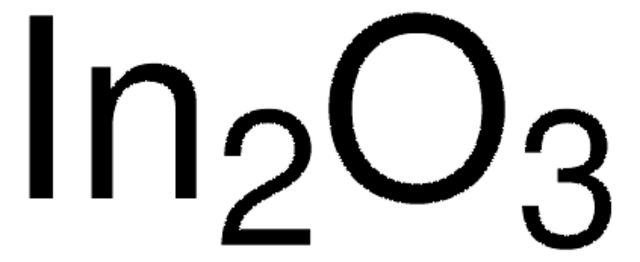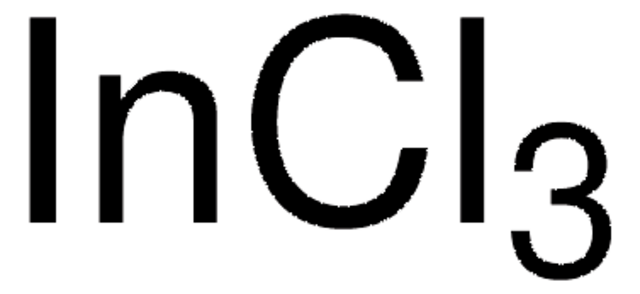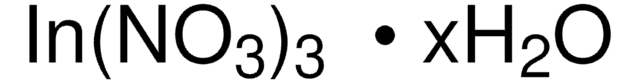203424
Indium(III) oxide
99.998% trace metals basis
Synonym(s):
Diindium trioxide, Indium sesquioxide
About This Item
Recommended Products
vapor pressure
<0.01 mmHg ( 25 °C)
Quality Level
assay
99.998% trace metals basis
form
powder
reaction suitability
reagent type: catalyst
core: indium
density
7.18 g/mL at 25 °C (lit.)
application(s)
battery manufacturing
SMILES string
O=[In]O[In]=O
InChI
1S/2In.3O
InChI key
SHTGRZNPWBITMM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Synthesis and Characterization: The development of gold nanoclusters on the surface of tin and indium oxide films, synthesizing new materials for advanced applications (Korotcenkov et al., 2014).
- Photocatalysis: Using nitrogen/sulfur-codoped carbon-coated indium oxide nanoparticles as excellent photocatalysts, providing insights into environmental and energy applications (Sun et al., 2019).
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Self-Assembled Nanodielectrics (SANDs) for Unconventional Electronics
Spectral conversion for solar cells is an emerging concept in the field of photovoltaics, and it has the potential to increase significantly the efficiency of solar cells. Lanthanide ions are ideal candidates for spectral conversion, due to their high luminescence efficiencies and rich energy level structure that allows for great flexibility in the upconversion and downconversion of photons in a wide spectral region (NIR-VIS-UV).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service