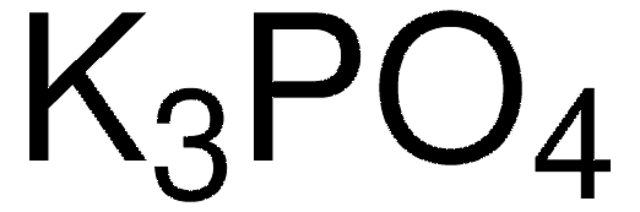205869
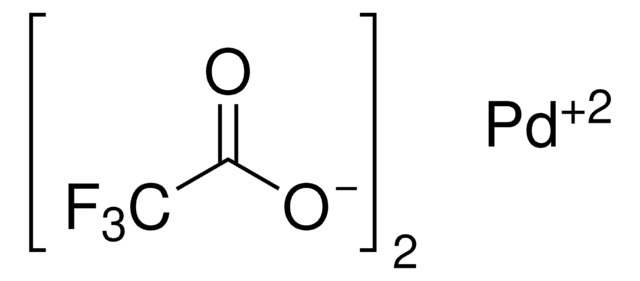
Palladium(II) acetate
reagent grade, 98%
Synonym(s):
Pd(OAc)2, [Pd(OAc)2]3
About This Item
Recommended Products
grade
reagent grade
Quality Level
assay
98%
form
powder
reaction suitability
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
mp
216.3-223.7 °C (dec.)
SMILES string
CC(O[Pd]OC(C)=O)=O
InChI
1S/2C2H4O2.Pd/c2*1-2(3)4;/h2*1H3,(H,3,4);/q;;+2/p-2
InChI key
YJVFFLUZDVXJQI-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Catalyst for the regioselective anti-hydrochlorination of the terminal and internal alkynes.
- Precursor to prepare a heterogeneous palladium complex catalyst for the Heck-Coupling reaction and Sonogashira cross-coupling reaction.
related product
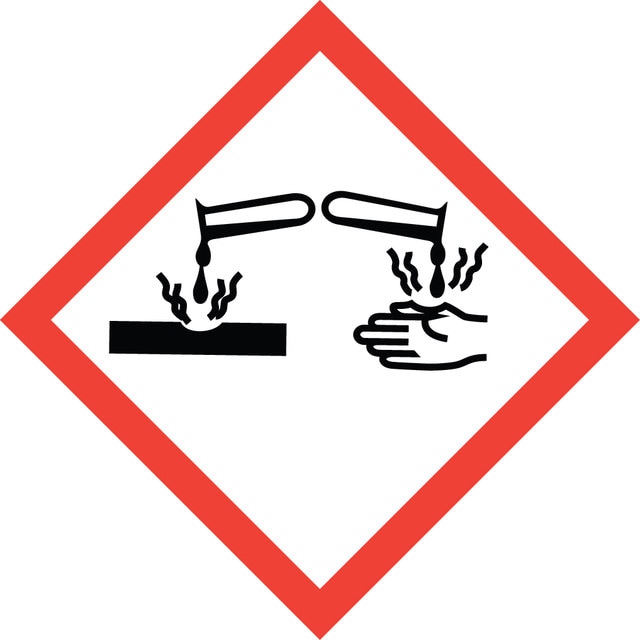
signalword
Danger
hcodes
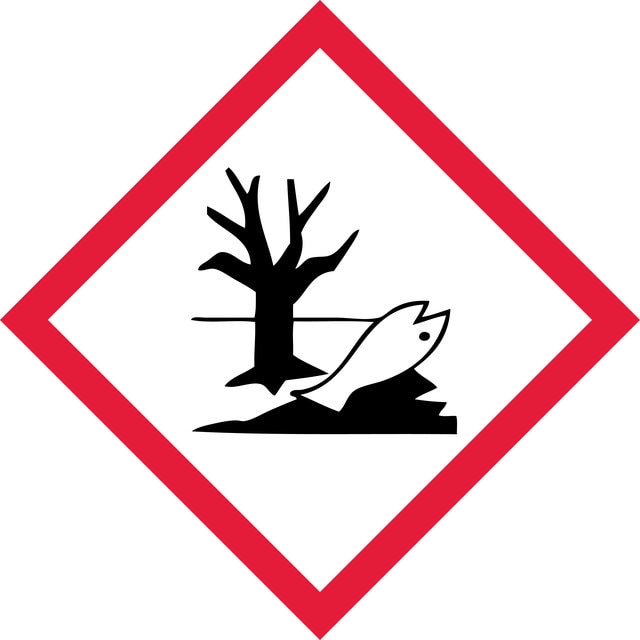
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Sens. 1A
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
A variety of transition-metal catalysts for the Suzuki coupling reaction are now available in our catalog. The majority of these catalysts are palladium- and nickelbased, typically utilizing phosphine-derived ligands.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service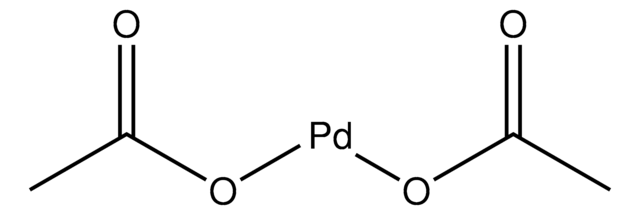
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)