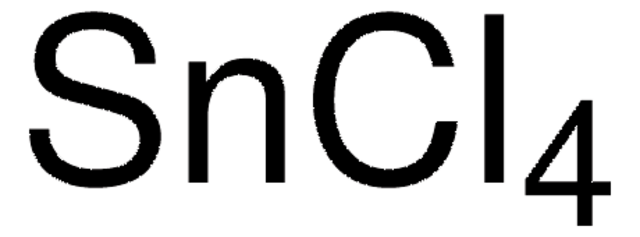208930
Tin(IV) chloride
98%
Synonym(s):
Stannic chloride fuming
About This Item
Recommended Products
vapor density
9 (vs air)
Quality Level
vapor pressure
10 mmHg ( 10 °C)
18.6 mmHg ( 20 °C)
20 mmHg ( 22 °C)
assay
98%
form
liquid
reaction suitability
core: tin
reagent type: Lewis acid
reagent type: catalyst
bp
114 °C (lit.)
mp
−33 °C (lit.)
density
2.226 g/mL at 25 °C (lit.)
SMILES string
Cl[Sn](Cl)(Cl)Cl
InChI
1S/4ClH.Sn/h4*1H;/q;;;;+4/p-4
InChI key
HPGGPRDJHPYFRM-UHFFFAOYSA-J
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Cyclization of trans β-monocyclohomofarnesic acid to form norambreinolide.
- Formation of C-glycosides of aromatic compounds such as thiophene, naphthalene and phloroglucinol trimethyl ether.
- Formal [4+2] cycloaddition of 3-ethoxycyclobutanones and allyltrialkylsilanes to form 3-ethoxy-5-[(trialkylsilyl)methyl]cyclohexan-1-ones.
- Diastereoselective reaction of δ-alkoxyallylstannanes with aldehydes to form 1,5-diol derivatives.
Other applications of SnCl4:
- The SnCl4-2,6-dialkoxyphenols complexes can catalyze cyclization of poylenes, such as 4-(homogeranyl)toluene to form trans-fused tricyclic compound.
- SnCl4 can act as a promoter during the reaction of ortho-aminobenzonitriles with β-ketoesters and β-enaminonitriles to form 4-aminoquinolines and 4-aminopyridines, respectively.
- SnCl4-silver perchlorate forms an effective catalytic system for the stereoselective of glycosylation 1-O-acetyl glucose.
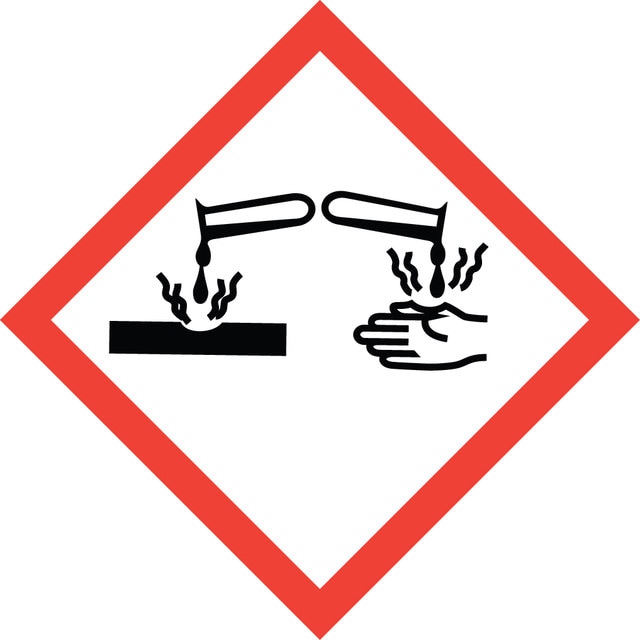
signalword
Danger
hcodes
Hazard Classifications
Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
target_organs
Respiratory system
Storage Class
8A - Combustible, corrosive hazardous materials
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service