All Photos(5)
About This Item
Empirical Formula (Hill Notation):
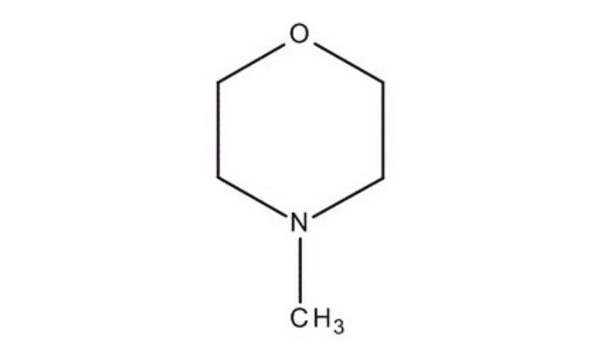
C5H11NO2
CAS Number:
Molecular Weight:
117.15
Beilstein/REAXYS Number:
507437
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
Recommended Products
Quality Level
assay
97%
reaction suitability
reagent type: oxidant
mp
180-184 °C (lit.)
storage temp.
2-8°C
SMILES string
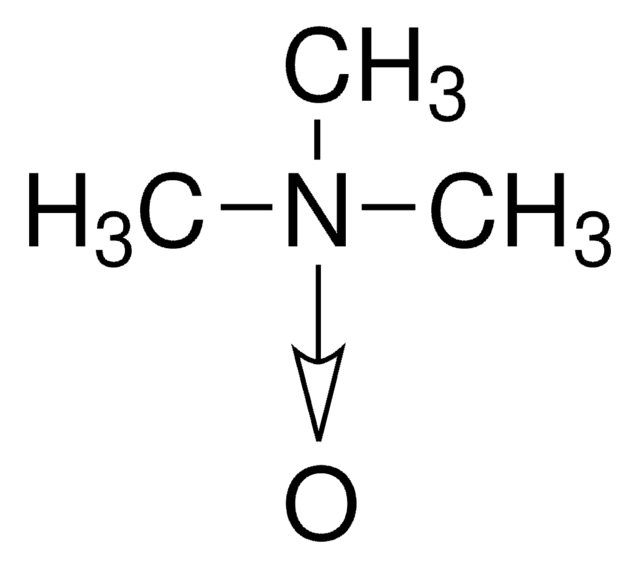
C[N+]1([O-])CCOCC1
InChI
1S/C5H11NO2/c1-6(7)2-4-8-5-3-6/h2-5H2,1H3
InChI key
LFTLOKWAGJYHHR-UHFFFAOYSA-N
Related Categories
General description
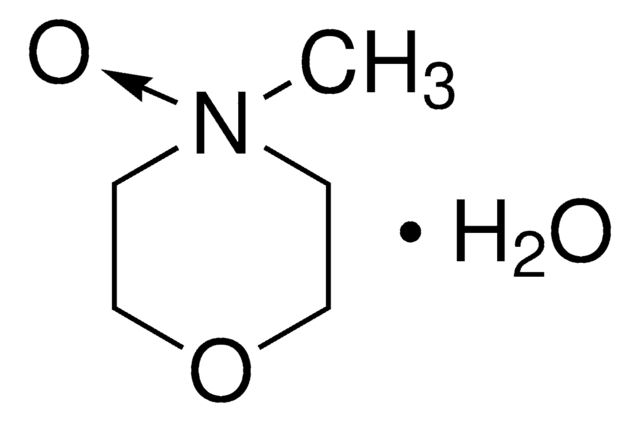
4-Methylmorpholine N-oxide is an organic compound used as a co-oxidant along with OsO4 and ruthenates in organic synthesis. In recent studies, it has been used as a catalyst in silylcyanation of aldehydes and ketones. Lyocell, a regenerated cellulose fiber, can be prepared using 4-methylmorpholine N-oxide in an eco-friendly manner.
Application
Non-metallic catalyst for the cyanosilylation of ketones. Co-oxidant for Sharpless asymmetric dihydroxylation in ionic liquids.
signalword
Danger
hcodes
Hazard Classifications
Flam. Sol. 1 - Repr. 2
Storage Class
4.1B - Flammable solid hazardous materials
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synlett, 6, 1077-1079 (2004)
Marzieh Shafiei et al.
Bioresource technology, 102(17), 7879-7886 (2011-06-21)
Given that N-methylmorpholine-N-oxide (NMMO) is a promising alternative for the pretreatment of lignocelluloses, a novel process for ethanol and biogas production from wood was developed. The solvent, NMMO, is concentrated by multistage evaporation, and the wood is pretreated with the
Nicolas Dupuy et al.
The Journal of chemical physics, 142(21), 214109-214109 (2015-06-08)
We study the ionization energy, electron affinity, and the π → π(∗) ((1)La) excitation energy of the anthracene molecule, by means of variational quantum Monte Carlo (QMC) methods based on a Jastrow correlated antisymmetrized geminal power (JAGP) wave function, developed
Luís C Branco et al.
The Journal of organic chemistry, 69(13), 4381-4389 (2004-06-19)
The use of room-temperature ionic liquids (RTILs) in the Sharpless catalytic asymmetric dihydroxylation (AD) as a cosolvent or replacement of the tert-butanol was studied in detail by screening 11 different RTILs. The AD reaction is faster in 1-n-butyl-3-methylimidazolium hexafluorophosphate [C(4)mim][PF(6)]
N?Methylmorpholine N?Oxide
Encyclopedia of Reagents for Organic Synthesis, Second Edition (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service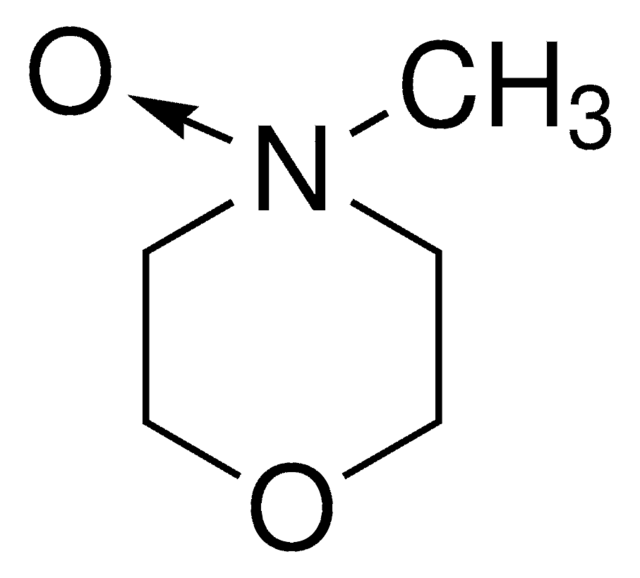


![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)