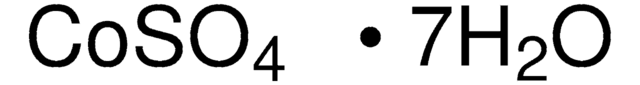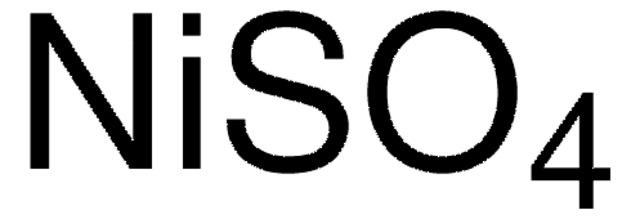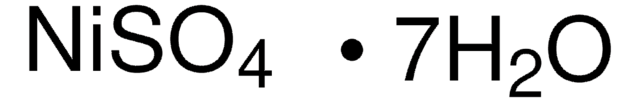229598
Cobalt(II) sulfate hydrate
99.998% trace metals basis
Synonym(s):
Cobaltous sulfate
About This Item
Recommended Products
Quality Level
assay
99.998% trace metals basis
form
crystals and lumps
impurities
≤25.0 ppm Trace Rare Earth Analysis
density
2.03 g/mL at 25 °C (lit.)
application(s)
battery manufacturing
SMILES string
O.[Co++].[O-]S([O-])(=O)=O
InChI
1S/Co.H2O4S.H2O/c;1-5(2,3)4;/h;(H2,1,2,3,4);1H2/q+2;;/p-2
InChI key
BGORGFZEVHFAQU-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
signalword
Danger
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B Inhalation - Muta. 2 - Repr. 1B - Resp. Sens. 1 - Skin Sens. 1
Storage Class
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
In many technologies, performance requirements drive device dimensions below the scale of electron mean free paths (λe). This trend has increased scientific interest and technological importance of electrical resistivities at the nanoscale.
Lithium-ion batteries represent a group of electrochemical devices used for electricity storage and have attracted a lot of attention in the past two decades due to their portability, rechargeability and low cost.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service