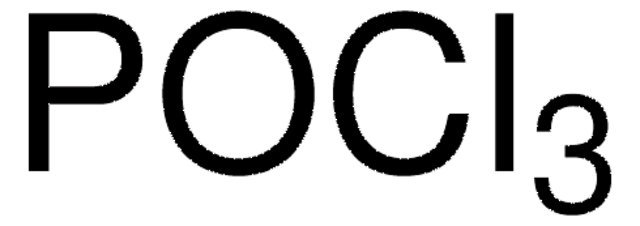All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H7NO2S
CAS Number:
Molecular Weight:
157.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
99%
form
solid
bp
100-102 °C/0.1 mmHg (lit.)
mp
62-64 °C (lit.)
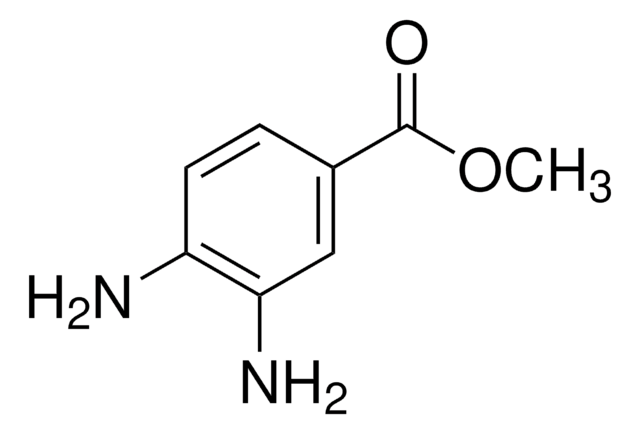
SMILES string
COC(=O)c1sccc1N
InChI
1S/C6H7NO2S/c1-9-6(8)5-4(7)2-3-10-5/h2-3H,7H2,1H3
InChI key
TWEQNZZOOFKOER-UHFFFAOYSA-N
Related Categories
General description
Methyl 3-amino-2-thiophenecarboxylate reacts with hydrazonoyl chlorides in the presence of triethylamine to yield corresponding N-arylamidrazones.
Application
Methyl 3-amino-2-thiophenecarboxylate was used in:
- the synthesis of 4-nitro and 4-aminothienyl ureas
- total synthesis of quinazolinocarboline alkaloids
- preparation of thienopyrimidinone analogs
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A short synthesis of quinazolinocarboline alkaloids rutaecarpine, hortiacine, euxylophoricine A and euxylophoricine D from methyl N-(4-chloro-5H-1, 2, 3-dithiazol-5-ylidene) anthranilates.
Mohanta PK and Kim K.
Tetrahedron Letters, 43(22), 3993-3996 (2002)
Donald L Hertzog et al.
Bioorganic & medicinal chemistry letters, 16(18), 4723-4727 (2006-07-28)
Optimization of a series of constrained melanin-concentrating hormone receptor 1 (MCH R1) antagonists has provided compounds with potent and selective MCH R1 activity. Details of the optimization process are provided and the use of one of the compounds in an
A M Redman et al.
Bioorganic & medicinal chemistry letters, 11(1), 9-12 (2001-01-05)
Inhibitors of the MAP kinase p38 are potentially useful for the treatment for osteoporosis, arthritis, and other inflammatory diseases. A series of thienyl, furyl, and pyrrolyl ureas has been identified as potent p38 inhibitors, displaying in vitro activity in the
Synthesis and Properties of Some New 1, 4-Dihydrothieno [3, 2-e][1, 2, 4] triazepin-5-ones.
Sabri SS, et al.
Zeitschrift fur Naturforschung, B: Chemical Sciences, 61(1), 65-65 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service