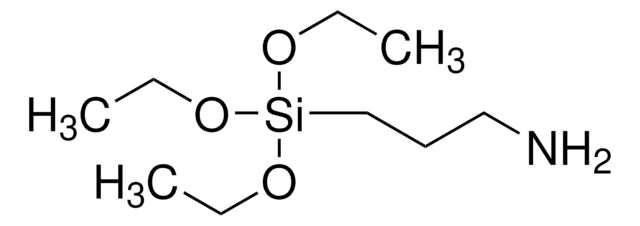
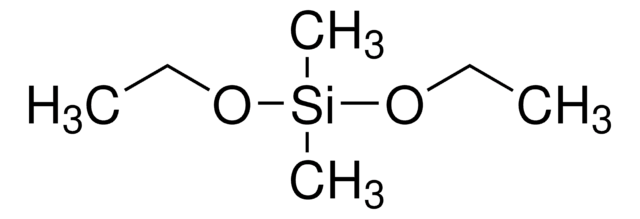
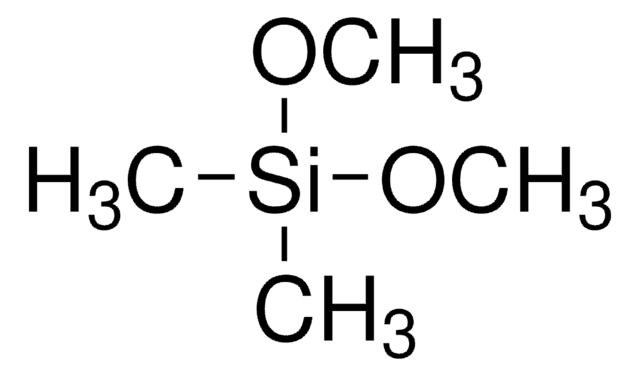
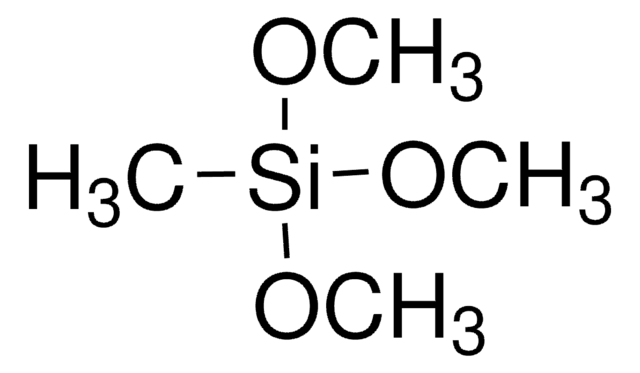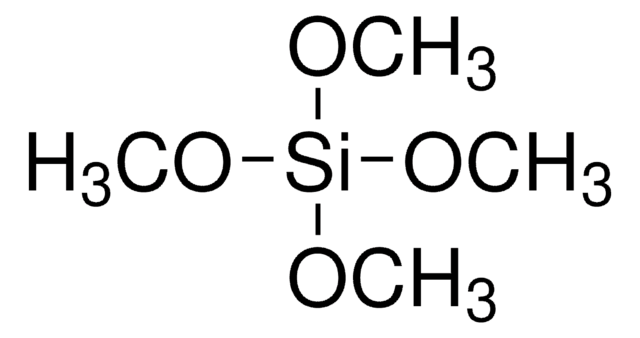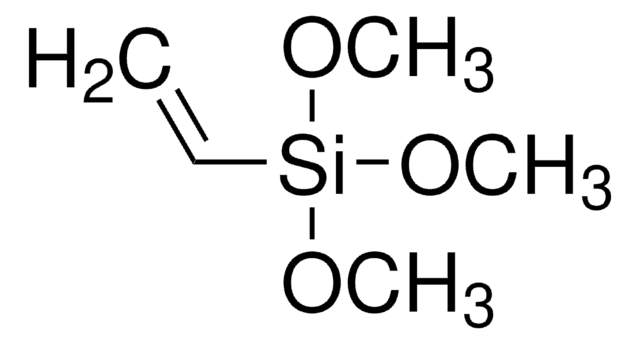246174
Trimethoxymethylsilane
98%
Synonym(s):
Methyltrimethoxysilane
About This Item
Recommended Products
Quality Level
assay
98%
form
liquid
refractive index
n20/D 1.371 (lit.)
bp
102-104 °C (lit.)
density
0.955 g/mL at 25 °C (lit.)
SMILES string
CO[Si](C)(OC)OC
InChI
1S/C4H12O3Si/c1-5-8(4,6-2)7-3/h1-4H3
InChI key
BFXIKLCIZHOAAZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- As a silica source for synthesizing polyethyleneimine-silica (PEI-silica) organic-inorganic hybrid particles.
- To transform hydrophilic ceramic surfaces to hydrophobic by modifying the -OH groups.
- To modify silica aerogels by inducing hydrophobicity and enhancing mechanical properties without affecting transparency.
signalword
Danger
hcodes
Hazard Classifications
Flam. Liq. 2
wgk_germany
WGK 3
flash_point_f
48.2 °F
flash_point_c
9 °C
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Advances in materials have often been led by the development of new synthetic methods that provide control over size, morphology and structure. The preparation of materials in a scalable and continuous manner is critical when development moves beyond lab-scale quantities.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service