All Photos(1)
About This Item
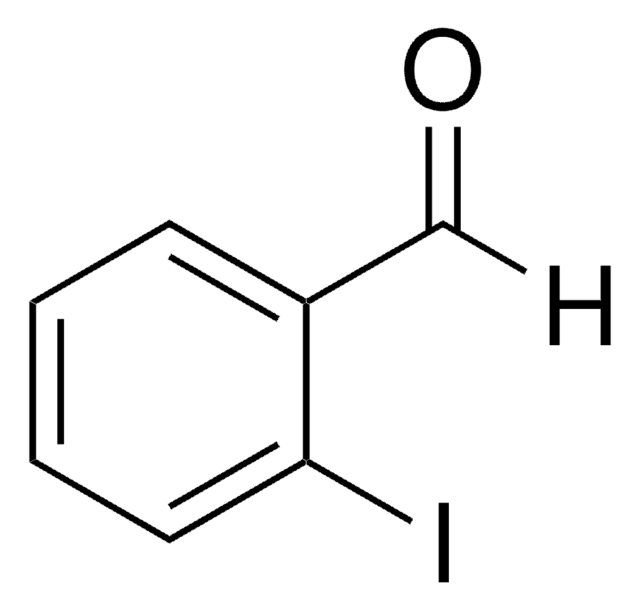
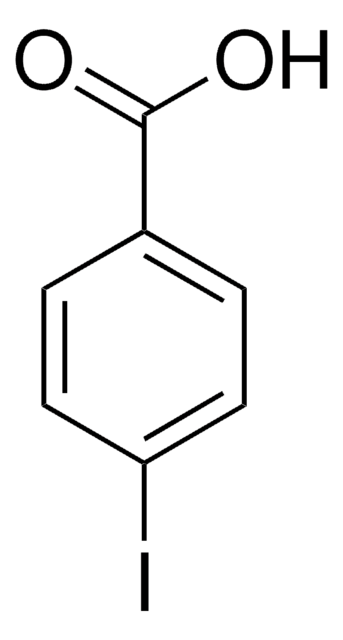
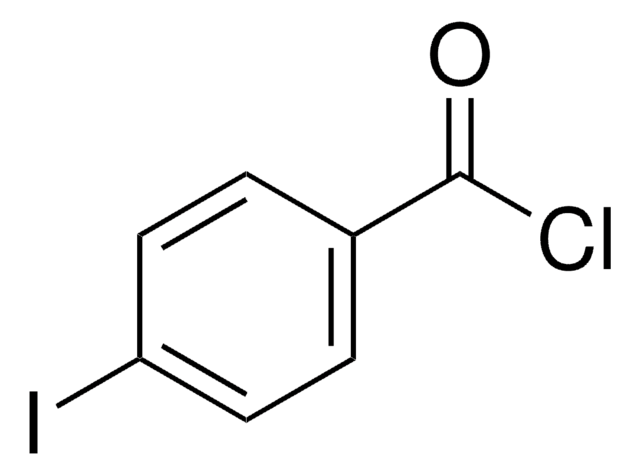
Linear Formula:
IC6H4CHO
CAS Number:
Molecular Weight:
232.02
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
96%
mp
78-82 °C (lit.)
storage temp.
2-8°C
SMILES string
Ic1ccc(C=O)cc1
InChI
1S/C7H5IO/c8-7-3-1-6(5-9)2-4-7/h1-5H
InChI key
NIEBHDXUIJSHSL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4-Iodobenzaldehyde participates in Suzuki–Miyaura coupling reaction to produce Metal–Organic Framework catalyst.
Application
4-Iodobenzaldehyde has been used in the preparation of:
- benzaldimine monolayers
- 4-[2-(trimethylsilyl)ethynyl]benzaldehyde
- 5,15-dimesityl-10-(3-[2-(trimethylsilyl)ethynyi]phenyl}-20-(4-iodophenyl)porphyrin
- 5,15-dimesityl-10-[3,5-bis{2-[4-(N,N′-difluoroboryl-1,9-dimethyidipyrrin-5-yl)-phenyl]ethynyl}phenyl]-20-(4-iodophenyl)porphyrin, multipigment building block
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Trans-Substituted porphyrin building blocks bearing iodo and ethynyl groups for applications in bioorganic and materials chemistry.
Ravikanth M, et al.
Tetrahedron, 54(27), 7721-7734 (1998)
Selective cleavage of the carbon-halide bond in substituted benzaldimine monolayers by synchrotron soft X-ray: Anomalously large cleavage rate of the carbon-bromide bond.
Moon JH, et al.
Langmuir, 16(6), 2981-2984 (2000)
Abel T Demissie et al.
Journal of the American Chemical Society, 137(27), 8819-8828 (2015-06-23)
We report the systematic characterization of anisotropic, π-conjugated oligophenyleneimine (OPI) films synthesized using stepwise imine condensation, or "click" chemistry. Film synthesis began with a self-assembled monolayer (SAM) of 4-formylthiophenol or 4-aminothiophenol on Au, followed by repetitive, alternate addition of terephthalaldehyde
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service