All Photos(2)
About This Item
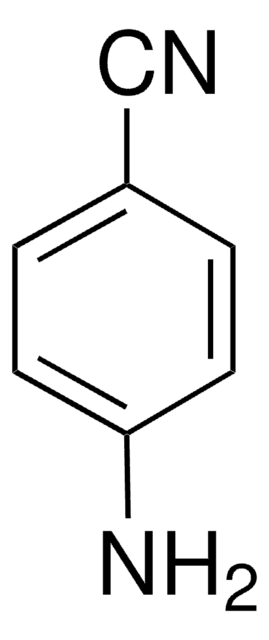
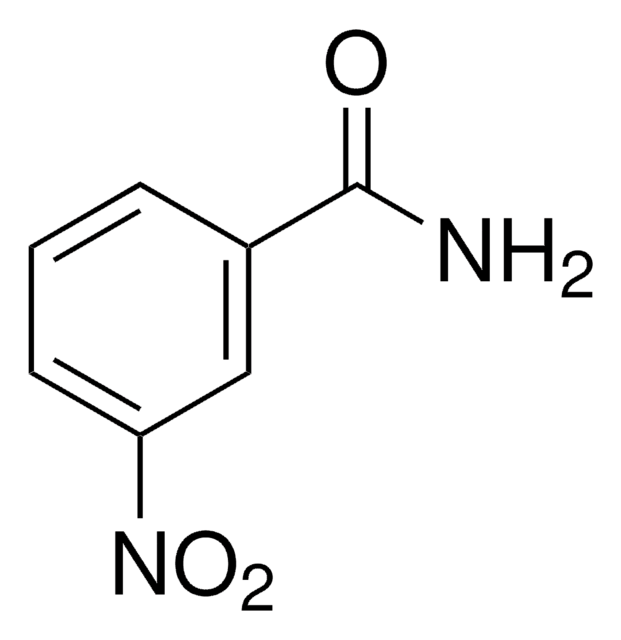
Linear Formula:
H2NC6H4CONH2
CAS Number:
Molecular Weight:
136.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
solid
mp
181-183 °C (lit.)
SMILES string
NC(=O)c1ccc(N)cc1
InChI
1S/C7H8N2O/c8-6-3-1-5(2-4-6)7(9)10/h1-4H,8H2,(H2,9,10)
InChI key
QIKYZXDTTPVVAC-UHFFFAOYSA-N
Related Categories
Application
4-Aminobenzamide was used as a poly(ADP-ribose)polymerase (PADPRP) inhhibitor to study the death of target cells by cytotoxic effector cells using the nuclear enzyme poly(ADP-ribose)polymerase (PADPRP).
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S D Ray et al.
Molecular and cellular biochemistry, 218(1-2), 27-33 (2001-05-02)
Previous studies from our laboratories have linked the protective abilities of IH636 grape seed proanthocyanidin extract (GSPE) with inactivation of anti-apoptotic gene bcl-XL, and modification of several other critical molecular targets such as DNA-damage/DNA-repair, lipid peroxidation and intracellular Ca2+ homeostasis.
Inhibition of nitric oxide induced cytotoxicity in pancreatic b-cells.
S A Brennan et al.
Biochemical Society transactions, 24(1), 73S-73S (1996-02-01)
S D Ray et al.
Free radical biology & medicine, 31(3), 277-291 (2001-07-20)
Acetaminophen (AAP), the analgesic hepatotoxicant, is a powerful inducer of oxidative stress, DNA fragmentation, and apoptosis. The anti-apoptotic oncogene bcl-XL, and the pro-apoptotic oncogene p53 are two key regulators of cell cycle progression and/or apoptosis subsequent to DNA damage in
R Laffranchi et al.
Experimental cell research, 237(1), 217-222 (1998-01-07)
Interferon-gamma is among the cytokines which have been implicated as effector molecules of beta-cell destruction in autoimmune diabetes. Its mechanism of action is, however, largely unknown. In the present study rat pancreatic beta-cells, INS-1, were incubated with rat interferon-gamma (rIRN-gamma)
D Monti et al.
Biochemical and biophysical research communications, 199(2), 525-530 (1994-03-15)
The death of target cells by cytotoxic effector cells is a relevant biological phenomenon, where cells are activated and a very quick apoptotic program occurs. In order to test the hypothesis that the nuclear enzyme poly(ADP-ribose)polymerase (PADPRP) plays a role
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service