296112
Diethylzinc solution
1.0 M in hexanes
Synonym(s):
Zincdiethyl
About This Item
Recommended Products
Quality Level
concentration
1.0 M in hexanes
density
0.726 g/mL at 25 °C
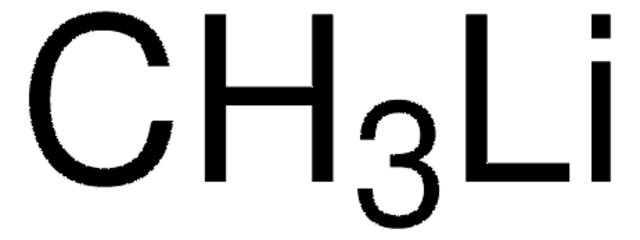
SMILES string
CC[Zn]CC
InChI
1S/2C2H5.Zn/c2*1-2;/h2*1H2,2H3;
InChI key
HQWPLXHWEZZGKY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- α-trifluoromethyl ketones from α, β-unsaturated ketones with Rh (I) catalyst.
- Alkynylzinc reagents which are used in cross-coupling reactions.
- Chiral secondary alcohols from aromatic and aliphatic aldehydes in presence of camphor-derived β-amino alcohol.
- 1, 4 conjugate addition product with enones.
- α-fluoroacrylates from aldehydes and ketones.
signalword
Danger
Hazard Classifications
Aquatic Chronic 2 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Pyr. Liq. 1 - Repr. 2 - Skin Corr. 1B - STOT RE 2 Inhalation - STOT SE 3 - Water-react 1
target_organs
Central nervous system, Nervous system
supp_hazards
Storage Class
4.2 - Pyrophoric and self-heating hazardous materials
wgk_germany
WGK 3
flash_point_f
-9.4 °F - closed cup
flash_point_c
-23 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service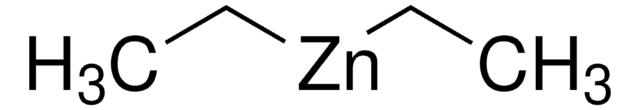





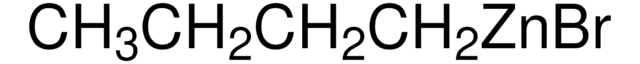
![(S,S)-(+)-2,6-Bis[2-(hydroxydiphenylmethyl)-1-pyrrolidinyl-methyl]-4-methylphenol 95%](/deepweb/assets/sigmaaldrich/product/structures/126/939/bff1a61c-8335-434f-8da4-9b1929aef17f/640/bff1a61c-8335-434f-8da4-9b1929aef17f.png)