All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H8O3
CAS Number:
Molecular Weight:
128.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
form
solid
mp
67-69 °C (lit.)
solubility
dichloromethane: soluble 25 mg/mL, clear, colorless to yellow
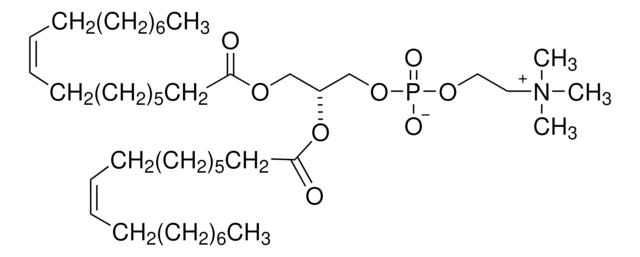
SMILES string
CC1(C)COC(=O)C1=O
InChI
1S/C6H8O3/c1-6(2)3-9-5(8)4(6)7/h3H2,1-2H3
InChI key
HRTOQFBQOFIFEE-UHFFFAOYSA-N
Related Categories
General description
Dihydro-4,4-dimethyl-2,3-furandione is an activated keto compound and its enantioselective hydrogenation was reported. Neutral Rhodium (I) aminophosphine-phosphinite complex calatyzed asymmetric hydrogenation of dihydro-4,4-dimethyl-2,3-furandione was reported. Asymmetric hydrogenation of dihydro-4,4-dimethyl-2,3-furandione gives D-(-)-pantoyl lactone, a key intermediate in the synthesis of pantothenic acid.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Norberto Bonalumi et al.
Journal of the American Chemical Society, 125(44), 13342-13343 (2003-10-30)
The combination of ATR-IR and modulation spectroscopy allowed for the study of the interaction of ketopantolactone with Pt/Al2O3 films chirally modified by cinchonidine under hydrogenation conditions. The spectra reveal a significant influence of ketopantolactone on the adsorption of the modifier
Rhodium (I) bis (aminophosphane) complexes as catalysts for asymmetric hydrogenation of activated ketones.
Roucoux A, et al.
Tetrahedron Asymmetry, 7(2), 379-382 (1996)
Organic Syntheses, 63, 18-18 (1985)
S Shimizu et al.
European journal of biochemistry, 174(1), 37-44 (1988-05-16)
A novel enzyme which specifically catalyzes the reduction of conjugated polyketones was purified to homogeneity from cells of Mucor ambiguus AKU 3006. The enzyme has a strict requirement for NADPH and irreversibly reduces a number of quinones such as p-benzoquinone
Neutral Rhodium (I) Aminophosphine-Phosphinite Complexes: Synthesis, Structure, and Use in Catalytic Asymmetric Hydrogenation of Activated Keto Compounds.
Agbossou F, et al.
Organometallics, 14(5), 2480-2489 (1995)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[(1,1-Dimethyl-2-propynyl)oxy]trimethylsilane 98%](/deepweb/assets/sigmaaldrich/product/structures/122/875/626ec472-06c3-453d-bcad-ce2e25d2b050/640/626ec472-06c3-453d-bcad-ce2e25d2b050.png)