34072
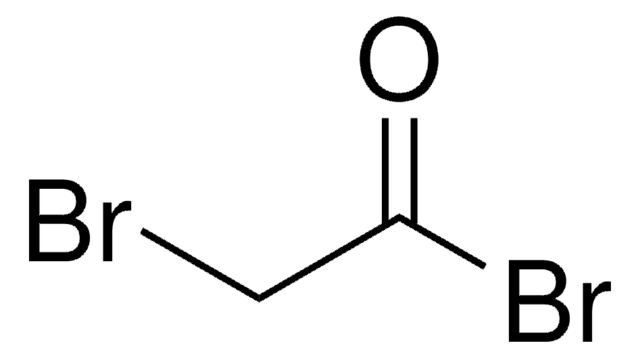
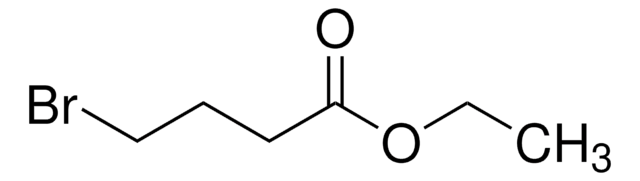
2,4-Dibromobutyryl chloride
technical, ~90% (GC)
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
BrCH2CH2CHBrCOCl
CAS Number:
Molecular Weight:
264.34
Beilstein/REAXYS Number:
4955401
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical
assay
~90% (GC)
refractive index
n20/D 1.535
density
2.00 g/mL at 20 °C
storage temp.
2-8°C
SMILES string
ClC(=O)C(Br)CCBr
InChI
1S/C4H5Br2ClO/c5-2-1-3(6)4(7)8/h3H,1-2H2
InChI key
WYZLYWUZERABRL-UHFFFAOYSA-N
Related Categories
Application
2,4-Dibromobutyryl chloride was used in the preparation of:
- methyl 2,4-dibromobutanoate
- series of 3-(3, 5-di-tert-butyl-4-hydroxybenzylidene)pyrrolidin-2-ones, anti-inflammatory/analgesic agents
- α-bromolactam
signalword
Danger
hcodes
Hazard Classifications
Resp. Sens. 1 - Skin Corr. 1B - Skin Sens. 1
supp_hazards
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Preparation of enantiopure 2-acylazetidines and their reactions with chloroformates.
Ma S, et al.
Tetrahedron Letters, 48(2), 269-271 (2007)
Bhooma Raghavan et al.
The Journal of organic chemistry, 71(5), 2151-2154 (2006-02-25)
A concise, stereoselective synthesis of alpha-substituted gamma-lactams is reported. gamma-Lactam scaffolds 2 and 3, possessing an Evans' chiral auxiliary and two types of N substituents, were successfully alkylated with different electrophiles to give alpha-substituted gamma-lactams with reasonable diastereoselectivities. The use
H Ikuta et al.
Journal of medicinal chemistry, 30(11), 1995-1998 (1987-11-01)
A series of 3-(3,5-di-tert-butyl-4-hydroxybenzylidene)pyrrolidin-2-ones was synthesized and evaluated as candidate antiinflammatory/analgesic agents as well as dual inhibitors of prostaglandin and leukotriene synthesis. Some compounds that showed dual inhibitory activity were found to possess equipotent antiinflammatory activities to indomethacin, with reduced
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service