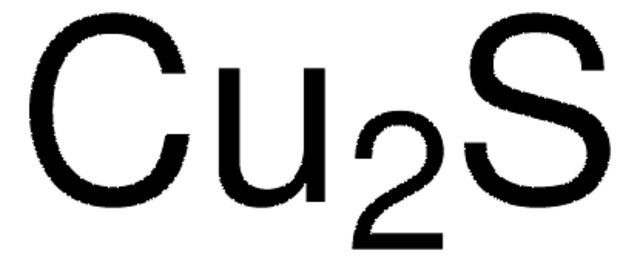342459
Copper(I) sulfide
powder, −325 mesh
Synonym(s):
Cuprous sulfide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
Cu2S
CAS Number:
Molecular Weight:
159.16
EC Number:
MDL number:
UNSPSC Code:
12352300
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
form
powder
Quality Level
particle size
−325 mesh
density
5.6 g/mL at 25 °C (lit.)
application(s)
battery manufacturing
SMILES string
[Cu]S[Cu]
InChI
1S/2Cu.S
InChI key
JESPAFKOCOFQIN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Copper sulfide shows metal-like electrical conductivity, chemical-sensing capability and suitable characteristics for absorption of solar energy. Cu63 NMR, X ray photoelectron spectroscopy,2 copper sulfide CuS was studied in detail. Copper sulfide is a monovalent and oxidation state was determined to be 2.2
Application
- Pharmacological Study on Ocfentanil: Research on the pharmacological properties of Ocfentanil, including its biochemistry and potent analgesic effects, was explored in a study where toxicology and behavioral impacts on zebrafish larvae and mice were assessed, confirming its potential for pain management applications (Bilel et al., 2023).
- Ocfentanil in Surgical Procedures: Ocfentanil′s role in surgical applications was highlighted in a study where new analytical methods were developed for its detection in biological samples, emphasizing its significance in pharmacological studies and surgical pain management (Jung et al., 2020).
- Ocfentanil Solution for Pharmacological Studies: The development and validation of a method for detecting Ocfentanil in hair samples indicate its critical role in forensic and pharmacological research, aiding in studies focused on its stability and effects within clinical settings (Allibe et al., 2021).
Packaging
Packaged in poly bottles
Other Notes
may contain a slight excess of S
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of copper sulfide nanorod arrays on molecular templates.
Mao G, et al.
Nano Letters, 4(2), 249-252 (2004)
Lidia Armelao et al.
Journal of nanoscience and nanotechnology, 6(2), 401-408 (2006-04-01)
A novel method for the preparation of CuS nanoparticles based on the fast nucleation of the sulphide has been developed. The particles have been synthesized by reaction of thioacetic acid with water and copper carboxylates (acetate, propionate) in the corresponding
Riccardo Narizzano et al.
The journal of physical chemistry. B, 109(33), 15798-15802 (2006-07-21)
A heterostructure formed by a conjugated polymer and semiconducting nanoparticles was produced. The conjugated polymer was synthesized by oxidative copolymerization of 3-thiopheneacetic acid and 3-hexylthiophene, thus obtaining an amphiphilic polythiophene that allows the formation of a stable polymer layer at
Yuming Guo et al.
Chemical communications (Cambridge, England), 46(20), 3493-3495 (2010-04-09)
Copper sulfide amorphous nanoparticles and nanocrystals were prepared successfully by a special process. These CuS nanoparticles could specifically and significantly induce the apoptosis and inhibit the proliferation of human cancer cells rather than normal cells. Moreover, the biological activities of
Xiangjie Bo et al.
Talanta, 81(1-2), 339-345 (2010-03-02)
A simple and facile synthetic method to incorporate copper sulfide (Cu(2)S) nanoparticles inside the mesopores of ordered mesoporous carbons (OMCs) is reported. The Cu(2)S/OMCs nanocomposite was characterized by transmission electron microscopy (TEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service