All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C8H5ClO3
CAS Number:
Molecular Weight:
184.58
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
99%
bp
155 °C/25 mmHg (lit.)
mp
78-79 °C (lit.)
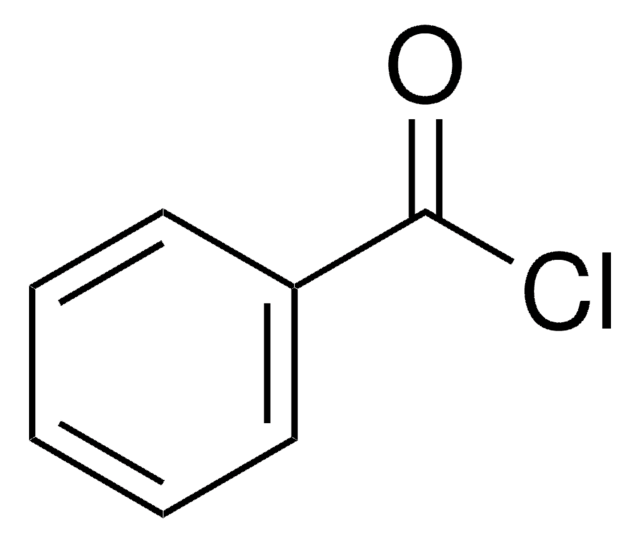
SMILES string
ClC(=O)c1ccc2OCOc2c1
InChI
1S/C8H5ClO3/c9-8(10)5-1-2-6-7(3-5)12-4-11-6/h1-3H,4H2
InChI key
ZRSGZIMDIHBXIN-UHFFFAOYSA-N
Related Categories
General description
Piperonyloyl chloride is an acyl halide. It participates in the preparation of starting reagent (N-acyl indole), required for the synthesis of pyrrolophenanthridone alkaloids. Kinetic study of the solvolysis of piperonyloyl chloride in various pure and binary solvent mixtures has been proposed. Solvolysis reaction has been reported to proceed via electron-rich acyl transfer mechanism.
Application
Piperonyloyl chloride is suitable for use in a kinetic study to evaluate the solvolysis rate constants of piperonyloyl chloride in 27 different solvents. It may be used in the synthesis of the following compounds:
- 2-phenylbenzimidazoles
- (Z)-3-hydroxy-1-(5-methoxy-2,2-dimethyl-2H-chromen-6-yl)-3-phenylprop-2-en-1-one
- pongapinone A
- 2-((1-(2-(N-(4-chlorophenyl)benzo[d][1,3]dioxole-5-carboxamido)ethyl)piperidin-4-yl)oxy)acetic acid phosphoric acid salt, inhibitor of platelet aggregation
- justicidin B, the piscicidal components of Justicia Hayatai var. decumbens
- piperazine derivatives
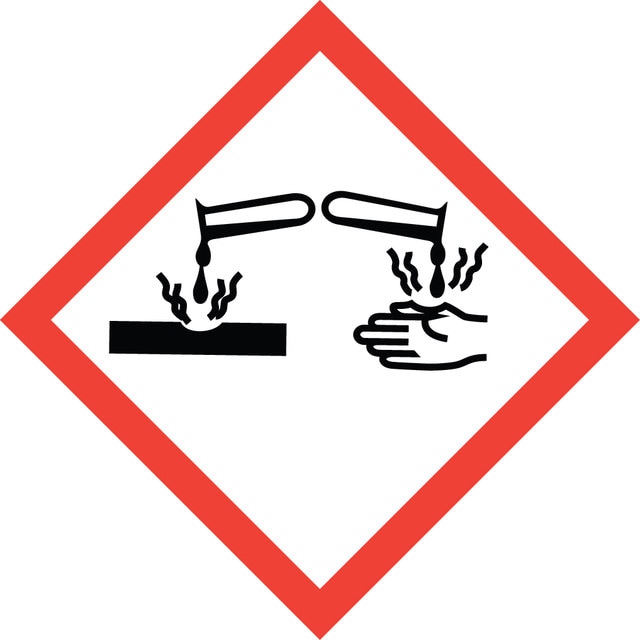
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Pelletier SW.
Alkaloids: Chemical and Biological Perspectives, Volume 14, 14, 441-442 (2000)
Studies on the Piscicidal Components of Justicia Hayatai var. decumbens.
OHTA K, et al.
Agricultural and Biological Chemistry, 33(4), 610-614 (1969)
Serena Scapecchi et al.
Bioorganic & medicinal chemistry, 12(1), 71-85 (2003-12-31)
Structure-activity relationships on two novel potent cognition enhancing drugs, unifiram (DM232, 1) and sunifiram (DM235, 2), are reported. Although none of the compounds synthesised reached the potency of the parent drugs, some fairly active compounds have been identified that may
Concise Synthetic Approaches to Naturally Occurring ?-Hydroxypyranochalcones: First Total Synthesis of Purpurenone, Its Derivative, and Praecansone B.
Wang X, et al.
Bull. Korean Chem. Soc., 33(8), 2647-2650 (2012)
Hachemi Kadri et al.
Journal of enzyme inhibition and medicinal chemistry, 23(5), 641-647 (2008-09-30)
A new series of fluorinated and non-fluorinated 2-phenylbenzimidazoles bearing oxygenated substituents on the phenyl ring has been synthesized. Synthesis of the new series was based on our previous discovery of 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (PMX 610) as a potent and selective antitumour agent
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service