390704
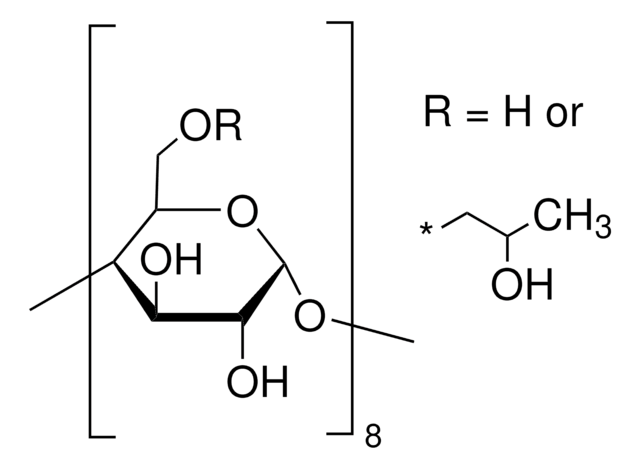
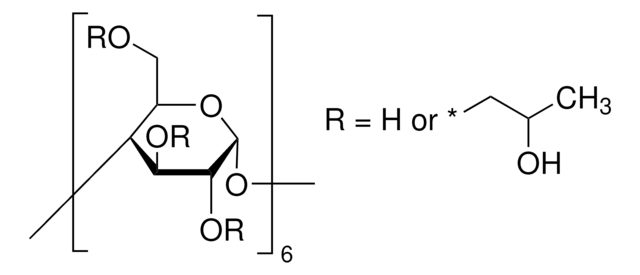
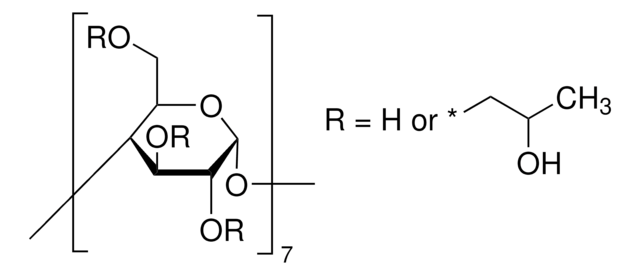
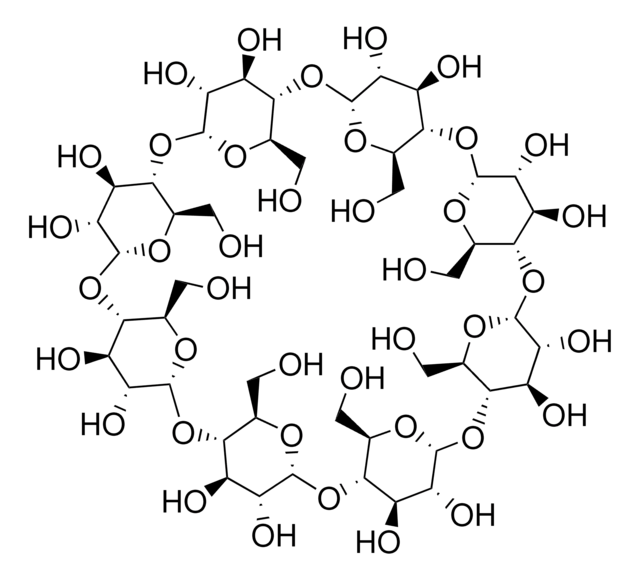
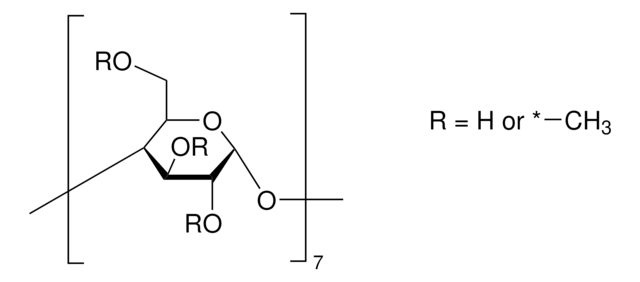
(2-Hydroxypropyl)-γ-cyclodextrin
extent of labeling: 0.6 molar substitution
Synonym(s):
HP-γ-CD, HPGCD, HGC
About This Item
Recommended Products
form
powder
Quality Level
optical activity
[α]20/D +145°, c = 1 in H2O
mol wt
average Mw ~1,580
extent of labeling
0.6 molar substitution
InChI
1S/C51H88O38/c1-14(56)8-73-11-21-42-29(64)36(71)50(81-21)85-40-19(6-54)79-48(34(69)27(40)62)89-44-23(13-75-10-16(3)58)82-51(37(72)30(44)65)86-41-20(7-55)78-47(33(68)26(41)61)88-43-22(12-74-9-15(2)57)80-49(35(70)28(43)63)84-39-18(5-53)76-45(31(66)24(39)59)83-38-17(4-52)77-46(87-42)32(67)25(38)60/h14-72H,4-13H2,1-3H3/t14?,15?,16?,17-,18-,19-,20-,21-,22-,23-,24-,25-,26-,27-,28-,29-,30-,31-,32-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-/m0/s1
InChI key
ODLHGICHYURWBS-RYJYQAAZSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- As a mobile phase additive in the study of the host-guest interaction with organic low molecular mass compounds prior to their quantification using reversed phase-high performance liquid chromatography (RP-HPLC) technique.
- As a chiral surfactant for the analysis of econazole by micellar electrokinetic chromatography (MEKC).
- As an analytical standard for the determination of the analyte in biological samples by HPLC.
- As a chiral selector for the identification of propiconazole by MEKC.
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service