All Photos(2)
About This Item
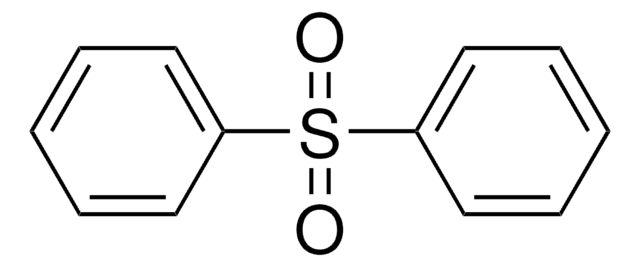
Linear Formula:
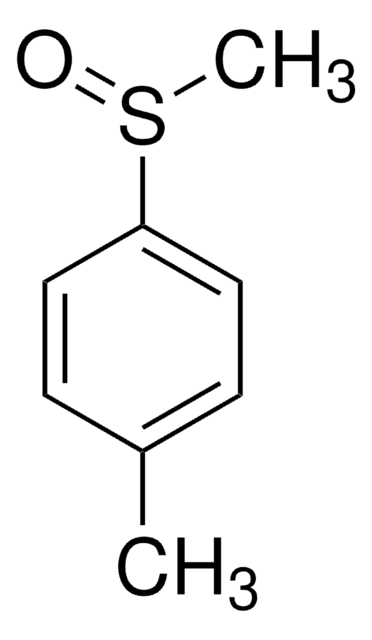
(CH3C6H4)2SO2
CAS Number:
Molecular Weight:
246.32
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
99%
form
solid
mp
158-160 °C (lit.)
solubility
chloroform: soluble 25 mg/mL, clear, colorless to yellow
SMILES string
Cc1ccc(cc1)S(=O)(=O)c2ccc(C)cc2
InChI
1S/C14H14O2S/c1-11-3-7-13(8-4-11)17(15,16)14-9-5-12(2)6-10-14/h3-10H,1-2H3
InChI key
WEAYCYAIVOIUMG-UHFFFAOYSA-N
Related Categories
General description
Di-p-tolyl sulfone is a di-p-substituted diaryl sulfone. Its synthesis by the sulfonylation of toluene with p-toluenesulfonic acid (TsOH) in the presence of polystyrene supported aluminium triflate (Ps-Al(OTf)3) catalyst has been reported along with its NMR and IR spectra. The gas-phase heats of formation of di-p-tolyl sulfone has been studied. The kinetics and thermodynamics of sulphuric acid assisted cleavage of di-p-tolyl sulfone has been invesitigated.
Application
Di-p-tolyl sulfone may be used in the synthesis of isomeric p-tolylpyridines (α , β and γ ) by photochemical decomposition.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Para tolylation of pyridine by photolysis of di-p-tolyl sulfone and related compounds.
Nakabayashi T, et al.
Bulletin of the Chemical Society of Japan, 50(9), 2491-2492 (1977)
Studies in the thermochemistry of sulphones. Part 6.-Heats of combustion, fusion, vaporization and atomization of six aromatic and two allylic sulphones.
Mackle H and O'Hare PAG.
Transactions of the Faraday Society, 57, 1521-1526 (1961)
Polystyrene Supported Al (OTf)3: a Stable, Efficient, Selective, and Reusable Catalyst for Sulfonylation of Arenes with Sulfonic Acids.
Boroujeni, KP.
Bull. Korean Chem. Soc., 31(7), 1887-1890 (2010)
Kinetics and thermodynamics of sulfuric acid-mediated cleavage of substituted diaryl sulfones.
Ward RS, et al.
Journal of Surfactants and Detergents, 4(2), 185-190 (2001)
Kazumasa Okamoto et al.
Scientific reports, 10(1), 19823-19823 (2020-11-15)
Dimer radical ions of aromatic molecules in which excess charge is localized in a pair of rings have been extensively investigated. While dimer radical cations of aromatics have been previously produced in the condensed phase, the number of molecules that
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service