449148
Potassium fluoride
anhydrous, powder, ≥99.9% trace metals basis
Synonym(s):
Potassium monofluoride, Potassium monofluoride (KF)
About This Item
Recommended Products
grade
anhydrous
Quality Level
assay
≥99.9% trace metals basis
form
powder
impurities
≤1000.0 ppm Trace Metal Analysis
mp
858 °C (lit.)
SMILES string
[F-].[K+]
InChI
1S/FH.K/h1H;/q;+1/p-1
InChI key
NROKBHXJSPEDAR-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- To fabricate CIGS thin films solar cells. Efficiency increased by up to 21.7% after KF postdeposition treatment. This was due to the formation of a K-containing layer on the CIGS surface, which resulted in increased surface reactivity due to the formation of a bilayer structure in which a K—In—Se species covers the CIGSe compound.
- To fabricate 2D vanadium carbide MXene for supercapacitor electrodes.
- To synthesize sulfonyl fluoride through electrochemical oxidative coupling.
- As an electrolyte additive for Li-ion batteries.
- As a catalyst for ring-opening fluorination of cyclic thioethers.
- To synthesize solid base catalysts, KF/Ca−Mg−Al hydrotalcite for the production of Biodiesel via transesterification reactions.
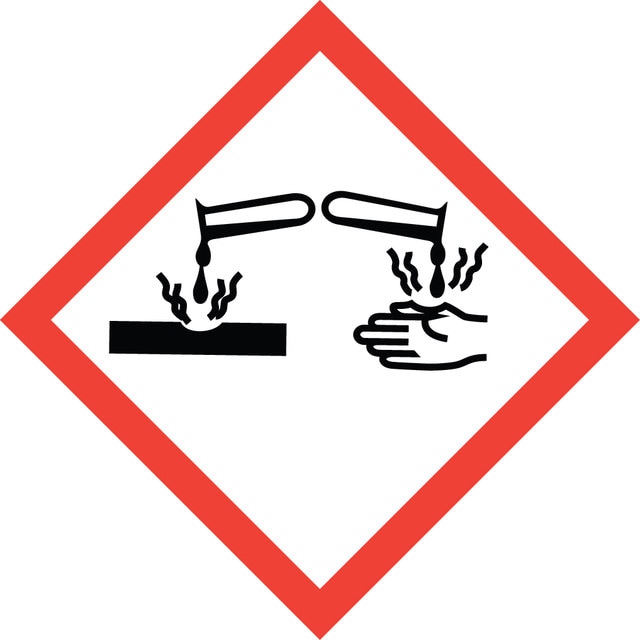
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Dam. 1
Storage Class
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) >95% in F+ active](/deepweb/assets/sigmaaldrich/product/structures/206/487/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d/640/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d.png)