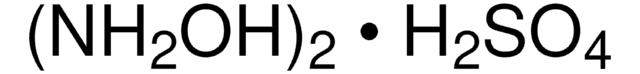467804
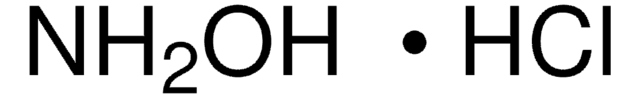
Hydroxylamine solution
50 wt. % in H2O, 99.999%
Synonym(s):
HDA
About This Item
Recommended Products
Quality Level
assay
99.999%
concentration
50 wt. % in H2O
bp
107 °C
density
1.078 g/mL at 25 °C
SMILES string
NO
InChI
1S/H3NO/c1-2/h2H,1H2
InChI key
AVXURJPOCDRRFD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Primary amides from aldehydes catalyzed by an arene–ruthenium(II) complex.
- Hydroxyaminoguanidines and carboxamide derivatives of ofloxacin for biological studies.
- Fe3O4/Au (GoldMag) nanoparticles for antibody immobilization.
- Prodrug for cardiovascular agent Nω-hydroxy-L-arginine (NOHA, nitric oxide precursor)
- Hydroxyaminoguanidines as anti-cancer agents
- Nonsteroidal 2,3-dihydroquinoline glucocorticoid receptor agonists with reduced phosphoenolpyruvate caboxykinase (PEPCK) transactivation
- Carboxamide derivatives of ofloxacin with improved antimicrobial properties
- Analogues of coumarin based TNF-α converting enzyme (TACE) inhibitors
- HIV integrase inhibitors
signalword
Danger
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Carc. 2 - Desen. Expl. 4 - Eye Dam. 1 - Met. Corr. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 2 - STOT SE 3
target_organs
Blood, Respiratory system
wgk_germany
WGK 3
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service